Novel Models for Chronic Intestinal Inflammation in Chickens: Intestinal Inflammation Pattern and Biomarkers
- PMID: 34054868
- PMCID: PMC8158159
- DOI: 10.3389/fimmu.2021.676628
Novel Models for Chronic Intestinal Inflammation in Chickens: Intestinal Inflammation Pattern and Biomarkers
Abstract
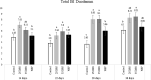
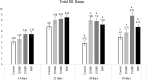
For poultry producers, chronic low-grade intestinal inflammation has a negative impact on productivity by impairing nutrient absorption and allocation of nutrients for growth. Understanding the triggers of chronic intestinal inflammation and developing a non-invasive measurement is crucial to managing gut health in poultry. In this study, we developed two novel models of low-grade chronic intestinal inflammation in broiler chickens: a chemical model using dextran sodium sulfate (DSS) and a dietary model using a high non-starch polysaccharide diet (NSP). Further, we evaluated the potential of several proteins as biomarkers of gut inflammation. For these experiments, the chemical induction of inflammation consisted of two 5-day cycles of oral gavage of either 0.25mg DSS/ml or 0.35mg DSS/ml; whereas the NSP diet (30% rice bran) was fed throughout the experiment. At four times (14, 22, 28 and 36-d post-hatch), necropsies were performed to collect intestinal samples for histology, and feces and serum for biomarkers quantification. Neither DSS nor NSP treatments affected feed intake or livability. NSP-fed birds exhibited intestinal inflammation through 14-d, which stabilized by 36-d. On the other hand, the cyclic DSS-treatment produced inflammation throughout the entire experimental period. Histological examination of the intestine revealed that the inflammation induced by both models exhibited similar spatial and temporal patterns with the duodenum and jejunum affected early (at 14-d) whereas the ileum was compromised by 28-d. Calprotectin (CALP) was the only serum protein found to be increased due to inflammation. However, fecal CALP and Lipocalin-2 (LCN-2) concentrations were significantly greater in the induced inflammation groups at 28-d. This experiment demonstrated for the first time, two in vivo models of chronic gut inflammation in chickens, a DSS and a nutritional NSP protocols. Based on these models we observed that intestinal inflammation begins in the upper segments of small intestine and moved to the lower region over time. In the searching for a fecal biomarker for intestinal inflammation, LCN-2 showed promising results. More importantly, calprotectin has a great potential as a novel biomarker for poultry measured both in serum and feces.
Keywords: DSS; ISI index; calprotectin; gut health; lipocalin; models of intestinal inflammation.
Copyright © 2021 Dal Pont, Belote, Lee, Bortoluzzi, Eyng, Sevastiyanova, Khadem, Santin, Farnell, Gougoulias and Kogut.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

