Acute Hemolysis and Heme Suppress Anti-CD40 Antibody-Induced Necro-Inflammatory Liver Disease
- PMID: 34054870
- PMCID: PMC8149790
- DOI: 10.3389/fimmu.2021.680855
Acute Hemolysis and Heme Suppress Anti-CD40 Antibody-Induced Necro-Inflammatory Liver Disease
Abstract
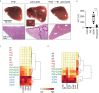
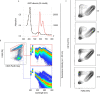
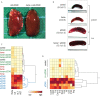
Clearance of red blood cells and hemoproteins is a key metabolic function of macrophages during hemolytic disorders and following tissue injury. Through this archetypical phagocytic function, heme is detoxified and iron is recycled to support erythropoiesis. Reciprocal interaction of heme metabolism and inflammatory macrophage functions may modify disease outcomes in a broad range of clinical conditions. We hypothesized that acute hemolysis and heme induce acute anti-inflammatory signals in liver macrophages. Using a macrophage-driven model of sterile liver inflammation, we showed that phenylhydrazine (PHZ)-mediated acute erythrophagocytosis blocked the anti-CD40 antibody-induced pathway of macrophage activation. This process attenuated the inflammatory cytokine release syndrome and necrotizing hepatitis induced by anti-CD40 antibody treatment of mice. We further established that administration of heme-albumin complexes specifically delivered heme to liver macrophages and replicated the anti-inflammatory effect of hemolysis. The anti-inflammatory heme-signal was induced in macrophages by an increased intracellular concentration of the porphyrin independently of iron. Overall, our work suggests that induction of heme-signaling strongly suppresses inflammatory macrophage function, providing protection against sterile liver inflammation.
Keywords: anti-CD40; erythrophagocytosis; heme; hepatitis; inflammation.
Copyright © 2021 Pfefferlé, Ingoglia, Schaer, Hansen, Schulthess, Humar, Schaer and Vallelian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

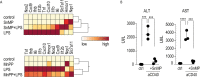
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

