Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn
- PMID: 34054875
- PMCID: PMC8158941
- DOI: 10.3389/fimmu.2021.683022
Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn
Abstract
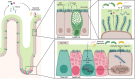
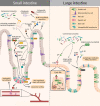
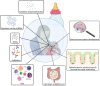
The innate immune system is the oldest protection strategy that is conserved across all organisms. Although having an unspecific action, it is the first and fastest defense mechanism against pathogens. Development of predominantly the adaptive immune system takes place after birth. However, some key components of the innate immune system evolve during the prenatal period of life, which endows the newborn with the ability to mount an immune response against pathogenic invaders directly after birth. Undoubtedly, the crosstalk between maternal immune cells, antibodies, dietary antigens, and microbial metabolites originating from the maternal microbiota are the key players in preparing the neonate's immunity to the outer world. Birth represents the biggest substantial environmental change in life, where the newborn leaves the protective amniotic sac and is exposed for the first time to a countless variety of microbes. Colonization of all body surfaces commences, including skin, lung, and gastrointestinal tract, leading to the establishment of the commensal microbiota and the maturation of the newborn immune system, and hence lifelong health. Pregnancy, birth, and the consumption of breast milk shape the immune development in coordination with maternal and newborn microbiota. Discrepancies in these fine-tuned microbiota interactions during each developmental stage can have long-term effects on disease susceptibility, such as metabolic syndrome, childhood asthma, or autoimmune type 1 diabetes. In this review, we will give an overview of the recent studies by discussing the multifaceted emergence of the newborn innate immune development in line with the importance of maternal and early life microbiota exposure and breast milk intake.
Keywords: birth; breast milk; early life; gestation; innate immune system; microbiota; neonate; pregnancy.
Copyright © 2021 Kalbermatter, Fernandez Trigo, Christensen and Ganal-Vonarburg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Maternal microbiota and antibodies as advocates of neonatal health.Gut Microbes. 2017 Sep 3;8(5):479-485. doi: 10.1080/19490976.2017.1299847. Epub 2017 Mar 1. Gut Microbes. 2017. PMID: 28296565 Free PMC article. Review.
-
The Impact of Maternal Microbes and Microbial Colonization in Early Life on Hematopoiesis.J Immunol. 2018 Apr 15;200(8):2519-2526. doi: 10.4049/jimmunol.1701776. J Immunol. 2018. PMID: 29632252 Review.
-
How maternal factors shape the immune system of breastfed infants to alleviate food allergy: A systematic and updated review.Immunology. 2025 Jan;174(1):1-16. doi: 10.1111/imm.13864. Epub 2024 Sep 30. Immunology. 2025. PMID: 39344356
-
Maternal milk and fecal microbes guide the spatiotemporal development of mucosa-associated microbiota and barrier function in the porcine neonatal gut.BMC Biol. 2019 Dec 18;17(1):106. doi: 10.1186/s12915-019-0729-2. BMC Biol. 2019. PMID: 31852478 Free PMC article.
-
Innate Immunity of Neonates and Infants.Front Immunol. 2018 Jul 30;9:1759. doi: 10.3389/fimmu.2018.01759. eCollection 2018. Front Immunol. 2018. PMID: 30105028 Free PMC article. Review.
Cited by
-
The source of microbial transmission influences niche colonization and microbiome development.Proc Biol Sci. 2024 Feb 14;291(2016):20232036. doi: 10.1098/rspb.2023.2036. Epub 2024 Feb 7. Proc Biol Sci. 2024. PMID: 38320611 Free PMC article.
-
Immunological factors influencing HIV acquisition in infants: A narrative review.Medicine (Baltimore). 2025 Jun 20;104(25):e43039. doi: 10.1097/MD.0000000000043039. Medicine (Baltimore). 2025. PMID: 40550044 Free PMC article. Review.
-
Can Vaginal Seeding at Birth Improve Health Outcomes of Cesarean Section-Delivered Infants? A Scoping Review.Microorganisms. 2025 May 28;13(6):1236. doi: 10.3390/microorganisms13061236. Microorganisms. 2025. PMID: 40572124 Free PMC article. Review.
-
Effects of Inducible Nitric Oxide Synthase (iNOS) Gene Knockout on the Diversity, Composition, and Function of Gut Microbiota in Adult Zebrafish.Biology (Basel). 2024 May 23;13(6):372. doi: 10.3390/biology13060372. Biology (Basel). 2024. PMID: 38927252 Free PMC article.
-
Impacts of Different Perinatal Factors on Faecal Immune Compounds in Infants: Determination of Normal Values.Int J Mol Sci. 2024 Oct 3;25(19):10675. doi: 10.3390/ijms251910675. Int J Mol Sci. 2024. PMID: 39409004 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

