STINGing the Tumor Microenvironment to Promote Therapeutic Tertiary Lymphoid Structure Development
- PMID: 34054879
- PMCID: PMC8155498
- DOI: 10.3389/fimmu.2021.690105
STINGing the Tumor Microenvironment to Promote Therapeutic Tertiary Lymphoid Structure Development
Abstract
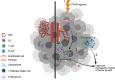
Tertiary lymphoid structures (TLS), also known as ectopic lymphoid structures (ELS) or tertiary lymphoid organs (TLO), represent a unique subset of lymphoid tissues noted for their architectural similarity to lymph nodes, but which conditionally form in peripheral tissues in a milieu of sustained inflammation. TLS serve as regional sites for induction and expansion of the host B and T cell repertoires via an operational paradigm involving mature dendritic cells (DC) and specialized endothelial cells (i.e. high endothelial venules; HEV) in a process directed by TLS-associated cytokines and chemokines. Recent clinical correlations have been reported for the presence of TLS within tumor biopsies with overall patient survival and responsiveness to interventional immunotherapy. Hence, therapeutic strategies to conditionally reinforce TLS formation within the tumor microenvironment (TME) via the targeting of DC, vascular endothelial cells (VEC) and local cytokine/chemokine profiles are actively being developed and tested in translational tumor models and early phase clinical trials. In this regard, a subset of agents that promote tumor vascular normalization (VN) have been observed to coordinately support the development of a pro-inflammatory TME, maturation of DC and VEC, local production of TLS-inducing cytokines and chemokines, and therapeutic TLS formation. This mini-review will focus on STING agonists, which were originally developed as anti-angiogenic agents, but which have recently been shown to be effective in promoting VN and TLS formation within the therapeutic TME. Future application of these drugs in combination immunotherapy approaches for greater therapeutic efficacy is further discussed.
Keywords: STING agonists; T cells; dendritic cells; immunotherapy; tertiary lymphoid structures; tumor; vaccine; vascular normalization.
Copyright © 2021 Filderman, Appleman, Chelvanambi, Taylor and Storkus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment.J Immunother Cancer. 2021 Feb;9(2):e001906. doi: 10.1136/jitc-2020-001906. J Immunother Cancer. 2021. PMID: 33526609 Free PMC article.
-
Targeting Tertiary Lymphoid Structures for Tumor Immunotherapy.Methods Mol Biol. 2018;1845:275-286. doi: 10.1007/978-1-4939-8709-2_16. Methods Mol Biol. 2018. PMID: 30141019
-
Radiation drives tertiary lymphoid structures to reshape TME for synergized antitumour immunity.Expert Rev Mol Med. 2024 Oct 23;26:e30. doi: 10.1017/erm.2024.27. Expert Rev Mol Med. 2024. PMID: 39438247 Free PMC article. Review.
-
High Endothelial Venules: A Vascular Perspective on Tertiary Lymphoid Structures in Cancer.Front Immunol. 2021 Aug 17;12:736670. doi: 10.3389/fimmu.2021.736670. eCollection 2021. Front Immunol. 2021. PMID: 34484246 Free PMC article. Review.
-
Tertiary lymphoid structures in the era of cancer immunotherapy.Nat Rev Cancer. 2019 Jun;19(6):307-325. doi: 10.1038/s41568-019-0144-6. Nat Rev Cancer. 2019. PMID: 31092904 Review.
Cited by
-
The roles of tertiary lymphoid structures in chronic diseases.Nat Rev Nephrol. 2023 Aug;19(8):525-537. doi: 10.1038/s41581-023-00706-z. Epub 2023 Apr 12. Nat Rev Nephrol. 2023. PMID: 37046081 Free PMC article. Review.
-
Associations between tertiary lymphoid structure density and immune checkpoint inhibitor efficacy in solid tumors: systematic review and meta-analysis.Front Immunol. 2024 Oct 31;15:1414884. doi: 10.3389/fimmu.2024.1414884. eCollection 2024. Front Immunol. 2024. PMID: 39544934 Free PMC article.
-
Improving cancer immunotherapy by rationally combining oncolytic virus with modulators targeting key signaling pathways.Mol Cancer. 2022 Oct 12;21(1):196. doi: 10.1186/s12943-022-01664-z. Mol Cancer. 2022. PMID: 36221123 Free PMC article. Review.
-
Translational and oncologic significance of tertiary lymphoid structures in pancreatic adenocarcinoma.Front Immunol. 2024 Feb 1;15:1324093. doi: 10.3389/fimmu.2024.1324093. eCollection 2024. Front Immunol. 2024. PMID: 38361928 Free PMC article. Review.
-
PDE-stable 2'3'-cGAMP analogues, containing 5'-S-phosphorothioester linkage, as STING agonists.RSC Med Chem. 2024 Jan 9;15(5):1508-1514. doi: 10.1039/d3md00593c. eCollection 2024 May 22. RSC Med Chem. 2024. PMID: 38784462 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

