Plant-Produced Receptor-Binding Domain of SARS-CoV-2 Elicits Potent Neutralizing Responses in Mice and Non-human Primates
- PMID: 34054909
- PMCID: PMC8158422
- DOI: 10.3389/fpls.2021.682953
Plant-Produced Receptor-Binding Domain of SARS-CoV-2 Elicits Potent Neutralizing Responses in Mice and Non-human Primates
Abstract
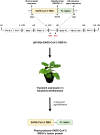
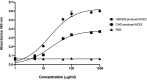
The emergence of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected global public health and economy. Despite the substantial efforts, only few vaccines are currently approved and some are in the different stages of clinical trials. As the disease rapidly spreads, an affordable and effective vaccine is urgently needed. In this study, we investigated the immunogenicity of plant-produced receptor-binding domain (RBD) of SARS-CoV-2 in order to use as a subunit vaccine. In this regard, RBD of SARS-CoV-2 was fused with Fc fragment of human IgG1 and transiently expressed in Nicotiana benthamiana by agroinfiltration. The plant-produced RBD-Fc fusion protein was purified from the crude extract by using protein A affinity column chromatography. Two intramuscular administration of plant-produced RBD-Fc protein formulated with alum as an adjuvant have elicited high neutralization titers in immunized mice and cynomolgus monkeys. Further it has induced a mixed Th1/Th2 immune responses and vaccine-specific T-lymphocyte responses which was confirmed by interferon-gamma (IFN-γ) enzyme-linked immunospot assay. Altogether, our results demonstrated that the plant-produced SARS-CoV-2 RBD has the potential to be used as an effective vaccine candidate against SARS-CoV-2. To our knowledge, this is the first report demonstrating the immunogenicity of plant-produced SARS-CoV-2 RBD protein in mice and non-human primates.
Keywords: COVID-19; Fc fusion protein; Nicotiana benthamiana; SARS-CoV-2; plant-produced recombinant protein; receptor-binding domain; subunit vaccine.
Copyright © 2021 Siriwattananon, Manopwisedjaroen, Shanmugaraj, Rattanapisit, Phumiamorn, Sapsutthipas, Trisiriwanich, Prompetchara, Ketloy, Buranapraditkun, Wijagkanalan, Tharakhet, Kaewpang, Leetanasaksakul, Kemthong, Suttisan, Malaivijitnond, Ruxrungtham, Thitithanyanont and Phoolcharoen.
Conflict of interest statement
WP from Chulalongkorn University is a founder/shareholder of Baiya Phytopharm Co., Ltd. BS and KR are employed by Baiya Phytopharm Co., Ltd., Thailand. WW is employed by BioNet-Asia Co., Ltd., Thailand. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern.J Med Virol. 2022 Sep;94(9):4265-4276. doi: 10.1002/jmv.27881. Epub 2022 Jun 1. J Med Virol. 2022. PMID: 35615895 Free PMC article.
-
Plant-Produced S1 Subunit Protein of SARS-CoV-2 Elicits Immunogenic Responses in Mice.Vaccines (Basel). 2022 Nov 18;10(11):1961. doi: 10.3390/vaccines10111961. Vaccines (Basel). 2022. PMID: 36423056 Free PMC article.
-
A recombinant subunit vaccine candidate produced in plants elicits neutralizing antibodies against SARS-CoV-2 variants in macaques.Front Plant Sci. 2022 Sep 28;13:901978. doi: 10.3389/fpls.2022.901978. eCollection 2022. Front Plant Sci. 2022. PMID: 36247553 Free PMC article.
-
Immunogenicity Studies of Plant-Produced SARS-CoV-2 Receptor Binding Domain-Based Subunit Vaccine Candidate with Different Adjuvant Formulations.Vaccines (Basel). 2021 Jul 5;9(7):744. doi: 10.3390/vaccines9070744. Vaccines (Basel). 2021. PMID: 34358160 Free PMC article.
-
Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines.Vaccines (Basel). 2021 Sep 6;9(9):992. doi: 10.3390/vaccines9090992. Vaccines (Basel). 2021. PMID: 34579229 Free PMC article. Review.
Cited by
-
Immunogenicity and efficacy of recombinant subunit SARS-CoV-2 vaccine candidate in the Syrian hamster model.Biotechnol Rep (Amst). 2023 Mar;37:e00779. doi: 10.1016/j.btre.2022.e00779. Epub 2022 Dec 13. Biotechnol Rep (Amst). 2023. PMID: 36533163 Free PMC article.
-
Development of SARS-CoV-2 neutralizing antibody detection assay by using recombinant plant-produced proteins.Biotechnol Rep (Amst). 2023 Jun;38:e00796. doi: 10.1016/j.btre.2023.e00796. Epub 2023 Apr 6. Biotechnol Rep (Amst). 2023. PMID: 37056791 Free PMC article.
-
Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern.J Med Virol. 2022 Sep;94(9):4265-4276. doi: 10.1002/jmv.27881. Epub 2022 Jun 1. J Med Virol. 2022. PMID: 35615895 Free PMC article.
-
Protein Expression Platforms and the Challenges of Viral Antigen Production.Vaccines (Basel). 2024 Nov 28;12(12):1344. doi: 10.3390/vaccines12121344. Vaccines (Basel). 2024. PMID: 39772006 Free PMC article. Review.
-
Rice-derived SARS-CoV-2 glycoprotein S1 subunit vaccine elicits humoral and cellular immune responses.Plant Biotechnol J. 2025 Jul;23(7):2570-2582. doi: 10.1111/pbi.70077. Epub 2025 Apr 4. Plant Biotechnol J. 2025. PMID: 40183251 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

