Patient-Controlled Analgesia (PCA): Intravenous Administration (IV-PCA) versus Oral Administration (Oral-PCA) by Using a Novel Device (PCoA® Acute) for Hospitalized Patients with Acute Postoperative Pain-A Comparative Retrospective Study
- PMID: 34055117
- PMCID: PMC8112920
- DOI: 10.1155/2021/2542010
Patient-Controlled Analgesia (PCA): Intravenous Administration (IV-PCA) versus Oral Administration (Oral-PCA) by Using a Novel Device (PCoA® Acute) for Hospitalized Patients with Acute Postoperative Pain-A Comparative Retrospective Study
Abstract
Background: Acute postoperative pain delays recovery and increases morbidity and mortality. Opioid therapy is effective but is accompanied by adverse reactions. Patient-controlled analgesia (PCA) enables self-administration of analgesics. Oral-PCA is a safe and beneficial alternative to intravenous (IV) PCA. We have developed a novel Oral-PCA device, which enables self-administration of solid pills to the patient's mouth. This is a retrospective study comparing the effectiveness and usability of this novel Oral-PCA with those of IV-PCA.
Methods: Medical records of patients who received PCA following gynecology and orthopedic surgeries were analyzed. The control cohort (n = 61) received oxycodone by IV-PCA. The test cohort (n = 44) received oxycodone by Oral-PCA via the PCoA Acute device. Outcome measures include the Numeric Rating Scale (NRS) score at rest and movement, side effects, technical difficulties, bolus dose administered, and bolus dose requested.
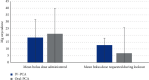
Results: Patient demographics, initial NRS, and PCA duration were comparable between cohorts. NRS reduction in rest and movement was stronger in the Oral-PCA cohort (rest: 1.61 and 2.27, P = 0.077; movement: 2.05 and 2.84, P = 0.039), indicating better pain control and mobility for Oral-PCA. Side effect rates were comparable between cohorts (9% and 11% of patients who experienced side effects, P = 1.000). The rate of technological difficulties was higher in the Oral-PCoA cohort (19.7% and 36.4%, P = 0.056). The mean total bolus dose administered to patients was comparable in both cohorts (18.32 mg and 21.14 mg oxycodone, P = 0.270). However, the mean total boluses requested by patients during lockout intervals were lower in the Oral-PCA cohort (12.8 mg and 6.82 mg oxycodone, P = 0.004), indicating better pain control.
Conclusions: Oral-PCA by using PCoA® Acute provides pain control and usability which is noninferior to the IV-PCA, as well as superior to pain reduction in rest and movement. These results, along with the noninvasiveness, medication flexibility, and reduced cost, suggest the potential of Oral-PCA, by using PCoA Acute, to replace IV-PCA for postoperative analgesia.
Copyright © 2021 Stefan Wirz et al.
Conflict of interest statement
The authors SW and SS declare that there are no conflicts of interest regarding the publication of this article. RS is the clinical and regulatory director at Dosentrx Ltd., the company that develops the PCoA Acute device and the ReX system.
Figures



Similar articles
-
The effectiveness of an oral opioid rescue medication algorithm for postoperative pain management compared to PCIA : A cohort analysis.Anaesthesist. 2020 Sep;69(9):639-648. doi: 10.1007/s00101-020-00806-6. Epub 2020 Jul 2. Anaesthesist. 2020. PMID: 32617631 Free PMC article.
-
Oral versus patient-controlled intravenous administration of oxycodone for pain relief after cesarean section.Arch Gynecol Obstet. 2019 Oct;300(4):903-909. doi: 10.1007/s00404-019-05260-3. Epub 2019 Aug 17. Arch Gynecol Obstet. 2019. PMID: 31422458 Free PMC article. Clinical Trial.
-
Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: A prospective, randomized, double-blind study.Medicine (Baltimore). 2017 Mar;96(10):e6286. doi: 10.1097/MD.0000000000006286. Medicine (Baltimore). 2017. PMID: 28272250 Free PMC article. Clinical Trial.
-
The Effectiveness of Intravenous Oxycodone in the Treatment of Acute Postoperative Pain: A Systematic Review.J Perianesth Nurs. 2018 Dec;33(6):865-879. doi: 10.1016/j.jopan.2017.05.010. Epub 2017 Oct 9. J Perianesth Nurs. 2018. PMID: 30449435
-
Perspectives on Intravenous Oxycodone for Control of Postoperative Pain.Pain Pract. 2016 Sep;16(7):924-34. doi: 10.1111/papr.12345. Epub 2015 Sep 22. Pain Pract. 2016. PMID: 26393529 Review.
Cited by
-
Optimizing CFTR modulator therapy management for cystic fibrosis through the ReX platform.Front Pediatr. 2023 Dec 19;11:1300968. doi: 10.3389/fped.2023.1300968. eCollection 2023. Front Pediatr. 2023. PMID: 38178914 Free PMC article.
-
Cost-effectiveness of the Perioperative Pain Management Bundle a registry-based study.Front Public Health. 2023 Sep 7;11:1157484. doi: 10.3389/fpubh.2023.1157484. eCollection 2023. Front Public Health. 2023. PMID: 37744520 Free PMC article.
-
Artificial intelligent patient-controlled intravenous analgesia improves the outcomes of older patients with laparoscopic radical resection for colorectal cancer.Eur Geriatr Med. 2023 Dec;14(6):1403-1410. doi: 10.1007/s41999-023-00873-z. Epub 2023 Oct 17. Eur Geriatr Med. 2023. PMID: 37847474 Free PMC article.
-
Knowledge, attitude and practice toward to artificial intelligent patient-controlled analgesia among anesthesiologists: a cross-sectional study in east China's Jiangsu Province.BMC Anesthesiol. 2024 Sep 20;24(1):335. doi: 10.1186/s12871-024-02724-1. BMC Anesthesiol. 2024. PMID: 39304835 Free PMC article.
-
Salivary cortisol as a biomarker of stress in surgical patients.J Med Biochem. 2023 Aug 25;42(3):469-475. doi: 10.5937/jomb0-42011. J Med Biochem. 2023. PMID: 37790204 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

