Tazarotene 0.045% Lotion for Moderate-to-Severe Acne in Male and Female Participants: A Phase II Post-hoc Analysis
- PMID: 34055190
- PMCID: PMC8142831
Tazarotene 0.045% Lotion for Moderate-to-Severe Acne in Male and Female Participants: A Phase II Post-hoc Analysis
Abstract
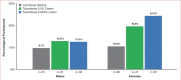
CLINICAL TRIALS ID: NCT02938494 BACKGROUND: In a Phase II study, tazarotene 0.045% lotion was statistically superior to vehicle and comparable to tazarotene 0.1% cream in reducing acne lesions, with fewer treatment-related adverse events (TEAEs) than the cream. OBJECTIVE: We analyzed data from the aforementioned study post-hoc to evaluate the effects of sex on treatment outcomes. METHODS: Participants aged 12 years or older with moderate-to-severe acne were randomized to tazarotene (0.045% lotion or 0.1% cream) or vehicle (lotion or cream) for 12 weeks of double-blind treatment. Outcomes analyzed in male and female subgroups included changes from baseline in inflammatory/noninflammatory lesions and TEAEs. RESULTS: In the intent-to-treat population (94 males and 116 females), reductions in lesion count were greater with tazarotene (lotion or cream) than with vehicle. In participants receiving tazarotene 0.045% lotion, the least-squares mean percent changes from baseline to Week 12 were greater in females than males, but the differences were not statistically significant (inflammatory [-70.3% vs. -56.2%]; noninflammatory [-60.0% vs. -53.2%]). In both females and males, the TEAE incidence was lower with tazarotene 0.045% lotion than 0.1% cream. CONCLUSION: Tazarotene 0.045% lotion substantially reduced acne lesions in both female and male participants. This newest tazarotene formulation might benefit patients who cannot tolerate older formulations or other topical retinoids. Given the relatively small size of this study, however, the results of this post-hoc analysis are intended to be exploratory in nature.
Keywords: Retinoid; acne; gender; sex; topical.
Copyright © 2021. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:This study was sponsored by Ortho Dermatologics. DISCLOSURES:Dr. Baldwin has served as advisor, investigator, and on the speakers’ bureaus for Almiral, Cassiopea, Foamix, Galderma, Ortho Dermatologics, Sol Gel, and Sun Pharma. Dr. Green has served as investigator, consultant, or speaker for Almirall, Cassiopea, Galderma, Ortho Dermatologics, Sol Gel, Sun Pharma, and Vyne. Dr. Kircik has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Dr. Guenin is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company. Ms. Forest and Dr. Pillai are employees of Bausch Health US, LLC, and may hold stock and/or stock options in its parent company. Bausch Health US, LLC is an affiliate of Bausch Health Companies Inc. Ortho Dermatologics is a division of Bausch Health US, LLC.
Figures




References
LinkOut - more resources
Full Text Sources
