De Novo Design, Synthesis, and Mechanistic Evaluation of Short Peptides That Mimic Heat Shock Protein 27 Activity
- PMID: 34055216
- PMCID: PMC8155270
- DOI: 10.1021/acsmedchemlett.0c00609
De Novo Design, Synthesis, and Mechanistic Evaluation of Short Peptides That Mimic Heat Shock Protein 27 Activity
Abstract
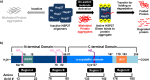
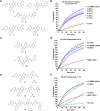
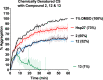
We report the first small molecule peptides based on the N-terminal sequence of heat shock protein 27 (Hsp27, gene HSPB1) that demonstrates chaperone-like activity. The peptide, comprising the SWDPF sequence located at Hsp27's amino (N)-terminal domain, directly regulates protein aggregation events, maintaining the disaggregated state of the model protein, citrate synthase. While traditional inhibitors of protein aggregation act via regulation of a protein that facilitates aggregation or disaggregation, our molecules are the first small peptides between 5 and 8 amino acids in length that are based on the N-terminus of Hsp27 and directly control protein aggregation. The presented strategy showcases a new approach for developing small peptides that control protein aggregation in proteins with high aggregate levels, making them a useful approach in developing new drugs.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
The N terminus of the small heat shock protein HSPB7 drives its polyQ aggregation-suppressing activity.J Biol Chem. 2019 Jun 21;294(25):9985-9994. doi: 10.1074/jbc.RA118.007117. Epub 2019 May 16. J Biol Chem. 2019. PMID: 31097540 Free PMC article.
-
Identification of peptides in human Hsp20 and Hsp27 that possess molecular chaperone and anti-apoptotic activities.Biochem J. 2015 Jan 1;465(1):115-25. doi: 10.1042/BJ20140837. Biochem J. 2015. PMID: 25332102 Free PMC article.
-
Analysis of the expression and function of the small heat shock protein gene, hsp27, in Xenopus laevis embryos.Comp Biochem Physiol A Mol Integr Physiol. 2007 May;147(1):112-21. doi: 10.1016/j.cbpa.2006.12.003. Epub 2006 Dec 12. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 17267255
-
The Hsp70 chaperone network.Nat Rev Mol Cell Biol. 2019 Nov;20(11):665-680. doi: 10.1038/s41580-019-0133-3. Nat Rev Mol Cell Biol. 2019. PMID: 31253954 Review.
-
The expression and function of hsp30-like small heat shock protein genes in amphibians, birds, fish, and reptiles.Comp Biochem Physiol A Mol Integr Physiol. 2017 Jan;203:179-192. doi: 10.1016/j.cbpa.2016.09.011. Epub 2016 Sep 17. Comp Biochem Physiol A Mol Integr Physiol. 2017. PMID: 27649598 Review.
Cited by
-
Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications.Polymers (Basel). 2023 Feb 25;15(5):1160. doi: 10.3390/polym15051160. Polymers (Basel). 2023. PMID: 36904404 Free PMC article. Review.
-
Anti-aggregation Properties of the Mini-Peptides Derived from Alpha Crystallin Domain of the Small Heat Shock Protein, Tpv HSP 14.3.Mol Biotechnol. 2024 Dec 8. doi: 10.1007/s12033-024-01332-1. Online ahead of print. Mol Biotechnol. 2024. PMID: 39645640
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

