Structural and in Vitro Functional Characterization of a Menthyl TRPM8 Antagonist Indicates Species-Dependent Regulation
- PMID: 34055223
- PMCID: PMC8155240
- DOI: 10.1021/acsmedchemlett.1c00001
Structural and in Vitro Functional Characterization of a Menthyl TRPM8 Antagonist Indicates Species-Dependent Regulation
Abstract
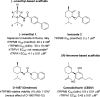
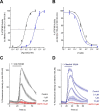
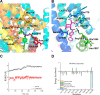
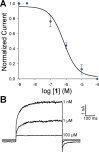
TRPM8 antagonists derived from its cognate ligand, (-)-menthol, are underrepresented. We determine the absolute stereochemistry of a well-known TRPM8 antagonist, (-)-menthyl 1, using VCD and 2D NMR. We explore 1 for its antagonist effects of the human TRPM8 (hTRPM8) orthologue to uncover species-dependent inhibition versus rat channels. (-)-Menthyl 1 inhibits menthol- and icilin-evoked Ca2+ responses at hTRPM8 with IC50 values of 805 ± 200 nM and 1.8 ± 0.6 μM, respectively, while more potently inhibiting agonist responses at the rat orthologue (rTRPM8 IC50 (menthol) = 117 ± 18 nM, IC50 (icilin) = 521 ± 20 nM). Whole-cell patch-clamp recordings of hTRPM8 confirm the 1 inhibition of menthol-stimulated currents, with an IC50 of 700 ± 200 nM. We demonstrate that 1 possesses ≥400-fold selectivity for hTRPM8 versus hTRPA1/hTRPV1. (-)-menthyl 1 can be used as a novel chemical tool to study hTRPM8 pharmacology and differences in species commonly used in drug discovery.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
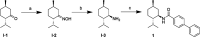




Similar articles
-
Structure-Based Design of Novel Biphenyl Amide Antagonists of Human Transient Receptor Potential Cation Channel Subfamily M Member 8 Channels with Potential Implications in the Treatment of Sensory Neuropathies.ACS Chem Neurosci. 2020 Feb 5;11(3):268-290. doi: 10.1021/acschemneuro.9b00404. Epub 2020 Jan 9. ACS Chem Neurosci. 2020. PMID: 31850745 Free PMC article.
-
Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives.J Biol Chem. 2009 Feb 13;284(7):4102-11. doi: 10.1074/jbc.M806651200. Epub 2008 Dec 18. J Biol Chem. 2009. PMID: 19095656
-
Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship.J Pharm Pharm Sci. 2010;13(2):242-53. doi: 10.18433/j3n88n. J Pharm Pharm Sci. 2010. PMID: 20816009
-
The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity.Mol Pain. 2010 Jan 21;6:4. doi: 10.1186/1744-8069-6-4. Mol Pain. 2010. PMID: 20092626 Free PMC article.
-
Inhibition of the cardiac L-type calcium channel current by the TRPM8 agonist, (-)-menthol.J Physiol Pharmacol. 2010 Oct;61(5):543-50. J Physiol Pharmacol. 2010. PMID: 21081797
Cited by
-
TRPM8 protein dynamics correlates with ligand structure and cellular function.bioRxiv [Preprint]. 2025 May 15:2025.05.13.653789. doi: 10.1101/2025.05.13.653789. bioRxiv. 2025. Update in: J Am Chem Soc. 2025 Jun 4;147(22):18460-18474. doi: 10.1021/jacs.4c09435. PMID: 40463232 Free PMC article. Updated. Preprint.
-
TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine.Nat Commun. 2022 Oct 22;13(1):6304. doi: 10.1038/s41467-022-33835-3. Nat Commun. 2022. PMID: 36272975 Free PMC article.
-
On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications.Front Oncol. 2023 Feb 9;12:1065935. doi: 10.3389/fonc.2022.1065935. eCollection 2022. Front Oncol. 2023. PMID: 36844925 Free PMC article. Review.
-
TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation.Int J Mol Sci. 2021 Aug 7;22(16):8502. doi: 10.3390/ijms22168502. Int J Mol Sci. 2021. PMID: 34445208 Free PMC article. Review.
-
L-Menthol-Loadable Electrospun Fibers of PMVEMA Anhydride for Topical Administration.Pharmaceutics. 2021 Nov 3;13(11):1845. doi: 10.3390/pharmaceutics13111845. Pharmaceutics. 2021. PMID: 34834260 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

