Development of Fluorescence Imaging Probes for Labeling COX-1 in Live Ovarian Cancer Cells
- PMID: 34055228
- PMCID: PMC8155275
- DOI: 10.1021/acsmedchemlett.1c00065
Development of Fluorescence Imaging Probes for Labeling COX-1 in Live Ovarian Cancer Cells
Abstract
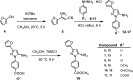
Recent experimental evidence demonstrated an aberrant overexpression of cyclooxygenase-1 (COX-1) in various cancers, which has stimulated the development of COX-1-selective inhibitors as promising anticancer drugs and cancer imaging agents. Herein we describe the synthesis and validation of 3-(furan-2-yl)-N-aryl 5-amino-pyrazoles as a novel class of COX-1 inhibitors, including molecular docking studies. Among all tested compounds, 4-(5-azido-3-(furan-2-yl)-1H-pyrazol-1-yl)benzoic 17 displayed a favorable COX-1 inhibition and selectivity profile (COX-1 IC50 = 0.1 μM, SI >1000 over COX-2). Compound 17 was selected as a lead structure for developing the novel COX-1-selective fluorescent probe 22. Fluorescent probe 22 was prepared via click chemistry by installing a nitro-benzoxadiazole motif as a fluorophore into the 3-(furan-2-yl)-N-aryl 5-amino-pyrazole scaffold. Fluorescence probe 22 was tested in ovarian cancer cell line OVCAR-3, confirming its usefulness for targeting and visualizing COX-1 in living cells with confocal microscopy.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



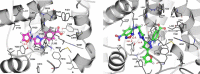
Similar articles
-
Fluorochrome Selection for Imaging Intraoperative Ovarian Cancer Probes.Pharmaceuticals (Basel). 2022 May 26;15(6):668. doi: 10.3390/ph15060668. Pharmaceuticals (Basel). 2022. PMID: 35745587 Free PMC article.
-
Fluorophore-labeled cyclooxygenase-2 inhibitors for the imaging of cyclooxygenase-2 overexpression in cancer: synthesis and biological studies.ChemMedChem. 2014 Jan;9(1):109-16, 240. doi: 10.1002/cmdc.201300355. Epub 2013 Oct 31. ChemMedChem. 2014. PMID: 24376205
-
Targeting COX-1 by mofezolac-based fluorescent probes for ovarian cancer detection.Eur J Med Chem. 2019 Oct 1;179:16-25. doi: 10.1016/j.ejmech.2019.06.039. Epub 2019 Jun 15. Eur J Med Chem. 2019. PMID: 31229884
-
Optimization of pyrazole-based compounds with 1,2,4-triazole-3-thiol moiety as selective COX-2 inhibitors cardioprotective drug candidates: Design, synthesis, cyclooxygenase inhibition, anti-inflammatory, ulcerogenicity, cardiovascular evaluation, and molecular modeling studies.Bioorg Chem. 2021 Sep;114:105122. doi: 10.1016/j.bioorg.2021.105122. Epub 2021 Jun 25. Bioorg Chem. 2021. PMID: 34243075
-
Design and synthesis of novel 2-phenyl-5-(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4-oxadiazoles as selective COX-2 inhibitors with potent anti-inflammatory activity.Eur J Med Chem. 2014 Jun 10;80:167-74. doi: 10.1016/j.ejmech.2014.04.045. Epub 2014 Apr 15. Eur J Med Chem. 2014. PMID: 24780593
Cited by
-
Fluorochrome Selection for Imaging Intraoperative Ovarian Cancer Probes.Pharmaceuticals (Basel). 2022 May 26;15(6):668. doi: 10.3390/ph15060668. Pharmaceuticals (Basel). 2022. PMID: 35745587 Free PMC article.
-
Fluorine-18 Labelled Radioligands for PET Imaging of Cyclooxygenase-2.Molecules. 2022 Jun 9;27(12):3722. doi: 10.3390/molecules27123722. Molecules. 2022. PMID: 35744851 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

