Termination of Repeat Testing in Chemical Laboratories Based on Practice Guidelines: Examining the Effect of Rule-Based Repeat Testing in a Transplantation Center
- PMID: 34055449
- PMCID: PMC8137285
- DOI: 10.1155/2021/9955990
Termination of Repeat Testing in Chemical Laboratories Based on Practice Guidelines: Examining the Effect of Rule-Based Repeat Testing in a Transplantation Center
Abstract
Background: Although the automation of instruments has reduced the variability of results and errors of analysis, in some laboratories, repeating a test to confirm its accuracy is still performed for critical and noncritical results. However, the importance of repeat testing is not well established yet, and there are no clear criteria for repeating a test.
Materials and methods: In this cross-sectional study, all repeated tests for 26 biochemical analytes (i.e., albumin, alkaline phosphatase (ALP), alanine aminotransferase (ALT), amylase, aspartate aminotransferase (AST), bilirubin total (BT), bilirubin direct (BD), blood urea nitrogen (BUN), calcium, chloride (Cl), cholesterol total (CholT), creatine kinase (CK), creatinine (Cr), glucose, gamma-glutamyl transferase (GGT), high-density lipoprotein-cholesterol (HDL-c), iron, lactate dehydrogenase (LDH), LDL-c, lipase, magnesium (Mg), phosphorus (Ph), protein total (ProtT), total iron binding capacity (TIBC), triglyceride (TG), and uric acid) were assessed in both critical and noncritical ranges over two consecutive months (routine subjective test repeats in the first month and rule-based repeats in the second month). To determine the usefulness of test repeats, differences between the initial and verified results were compared with the allowable bias, and repeat testing was considered necessary if it exceeded the allowable bias range. All causes of repeat testing, including linearity flags, delta checks, clinically significant values, and critical values, were also documented. All data, including the cause of repeats, initial and verified results, time, and costs in the two consecutive months, were transferred to Microsoft Excel for analysis. For comparison of data between the months, Student's t-test was used.
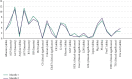
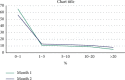
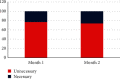
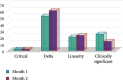
Results: A total of 7714 repeat tests were performed over two consecutive months. Although a significant decline (38%) was found in repeated tests in the second month (P < 0.001), there was no significant change in the percentage of unnecessary repeats (77% in the first month and 74% in the second month). In both consecutive months, AST and ALT were the most commonly repeated tests, and delta check was the most common cause of repeat testing. Mg, ALP, AST, and lipase showed the highest rates of necessary repeats, respectively (the least stable tests), while albumin, LDL, and CholT tests showed the highest rates of unnecessary repeats, respectively (the most stable tests). The total cost and delay in turnaround time (TAT) due to repeated testing decreased by 32% and 36%, respectively.
Conclusion: Although repeat testing has been shown to be unnecessary in most cases, having a strict policy for repeat testing appears to be more valuable than avoiding it completely. Each laboratory is advised to establish its own protocol for repeat testing based on its own practice.
Copyright © 2021 Neda Soleimani et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Derivation of sex and age-specific reference intervals for clinical chemistry analytes in healthy Ghanaian adults.Clin Chem Lab Med. 2022 Jul 4;60(9):1426-1439. doi: 10.1515/cclm-2022-0293. Print 2022 Aug 26. Clin Chem Lab Med. 2022. PMID: 35786502
-
Assessment of Sigma Metrics for Routine Chemistry Testing in 4 Laboratories in Kwa-Zulu Natal, South Africa.J Appl Lab Med. 2022 May 4;7(3):689-697. doi: 10.1093/jalm/jfab117. J Appl Lab Med. 2022. PMID: 34636901
-
Use of total patient data for indirect estimation of reference intervals for 40 clinical chemical analytes in Turkey.Clin Chem Lab Med. 2006;44(7):867-76. doi: 10.1515/CCLM.2006.139. Clin Chem Lab Med. 2006. PMID: 16776635
-
Does laboratory automation for the preanalytical phase improve data quality?J Lab Autom. 2013 Oct;18(5):375-81. doi: 10.1177/2211068213488892. Epub 2013 May 17. J Lab Autom. 2013. PMID: 23686657
-
The impact of pre-analytical variations on biochemical analytes stability: A systematic review.J Clin Lab Anal. 2020 Dec;34(12):e23551. doi: 10.1002/jcla.23551. Epub 2020 Sep 1. J Clin Lab Anal. 2020. PMID: 32869910 Free PMC article.
Cited by
-
Reducing the need for repeating urine drug testing with the gray zone determined by the measurement uncertainty.J Med Biochem. 2023 Oct 27;42(4):616-620. doi: 10.5937/jomb0-41777. J Med Biochem. 2023. PMID: 38084242 Free PMC article.
-
Comparison of Point-of-Care and Highly Sensitive Laboratory Troponin Testing in Patients Suspicious of Acute Myocardial Infarction and Its Efficacy in Clinical Outcome.Cardiol Res Pract. 2022 Feb 24;2022:6914979. doi: 10.1155/2022/6914979. eCollection 2022. Cardiol Res Pract. 2022. PMID: 35251711 Free PMC article.
References
-
- Burtis C. A., Bruns D. E., Sawyer B. G. Teitz Fundamental of Clinical Chemistry and Molecular Diagnostics. 7th. Philadeiphia, PA, USA: Saunders; 2015.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

