Profiling Humoral Immune Response Against Pre-Erythrocytic and Erythrocytic Antigens of Malaria Parasites Among Neotropical Primates in the Brazilian Atlantic Forest
- PMID: 34055672
- PMCID: PMC8155606
- DOI: 10.3389/fcimb.2021.678996
Profiling Humoral Immune Response Against Pre-Erythrocytic and Erythrocytic Antigens of Malaria Parasites Among Neotropical Primates in the Brazilian Atlantic Forest
Abstract
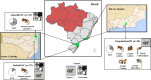
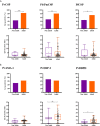
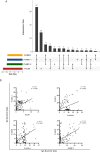
Human malaria due to zoonotic transmission has been recorded in the Atlantic Forest, an extra-Amazonian area in Brazil, which are a challenge for malaria control. Naturally acquired humoral immune response against pre-erythrocytic and erythrocytic antigens of Neotropical primates (NP) was evaluated here to improve the knowledge about the exposure of those animals to the malaria transmission and support the identification of the potential reservoirs of the disease in the Atlantic Forest. Blood samples of 154 monkeys from three areas of the Atlantic Forest were used to identify IgG antibodies against peptides of the repeat region of the major pre-erythrocytic antigen, the circumsporozoite protein (CSP), of Plasmodium vivax (PvCSP), Plasmodium brasilianum/Plasmodium malariae (Pb/PmCSP), and Plasmodium falciparum (PfCSP) by ELISA. Antibodies against erythrocytic recombinant antigens of P. vivax, Apical membrane antigen 1 (PvAMA-1), Erythrocyte binding protein 2 (PvEBP-2) and domain II of Duffy binding protein (PvDBPII) were also evaluated. Parameters, such as age, sex, PCR positivity, and captivity, potentially associated with humoral immune response were analyzed. Eighty-five percent of NP had antibodies against at least one CSP peptide, and 76% against at least one P. vivax erythrocytic antigen. A high percentage of adults compared to non-adults were seropositive and showed increased antibody levels. Neotropical primates with PCR positive for P. simium had a significantly higher frequency of positivity rate for immune response against PvEBP-2, PvDBPII and also higher antibody levels against PvDBPII, compared to PCR negative NPs for this species. Monkeys with PCR positive for P. brasilianum/P. malariae showed higher frequency of seropositivity and antibody levels against Pb/PmCSP. Levels of antibodies against Pb/PmCSP, PvEBP-2 and PvDBPII were higher in free-living than in captive monkeys from the same area. All Platyrrhine families showed antibodies against CSP peptides, however not all showed IgG against erythrocytic antigens. These findings showed a high prevalence of naturally acquired antibodies against CSP repeats in all studied areas, suggesting an intense exposure to infected-mosquitoes bites of NP from all families. However, mainly monkeys of Atelidae family showed antibodies against P. vivax erythrocytic antigens, suggesting blood infection, which might serve as potential reservoirs of malaria in the Atlantic Forest.
Keywords: Atlantic forest; erythrocytic antigens; humoral response; malaria; neotropical primates; pre-erythrocytic antigen.
Copyright © 2021 Assis, Alvarenga, Costa Pereira, Sánchez-Arcila, de Pina Costa, Souza Junior, Nunes, Pissinatti, Moreira, de Menezes Torres, Costa, da Penha Tinoco, Pereira, Soares, Sousa, Ntumngia, Adams, Kano, Hirano, Daniel-Ribeiro, Oliveira Ferreira, Carvalho and Alves de Brito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer TO declared a shared affiliation, with no collaboration, with one of the authors, IS, to the handling editor at the time of review.
Figures




Similar articles
-
IgM antibody responses against Plasmodium antigens in neotropical primates in the Brazilian Atlantic Forest.Front Cell Infect Microbiol. 2023 Sep 27;13:1169552. doi: 10.3389/fcimb.2023.1169552. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37829607 Free PMC article.
-
Malaria epidemiology in low-endemicity areas of the Atlantic Forest in the Vale do Ribeira, São Paulo, Brazil.Acta Trop. 2006 Nov;100(1-2):54-62. doi: 10.1016/j.actatropica.2006.09.010. Acta Trop. 2006. PMID: 17126279
-
Detection of etiological agents of malaria in howler monkeys from Atlantic Forests, rescued in regions of São Paulo city, Brazil.J Med Primatol. 2011 Dec;40(6):392-400. doi: 10.1111/j.1600-0684.2011.00498.x. Epub 2011 Sep 20. J Med Primatol. 2011. PMID: 21933192
-
Plasmodium vivax Duffy Binding Protein-Based Vaccine: a Distant Dream.Front Cell Infect Microbiol. 2022 Jul 13;12:916702. doi: 10.3389/fcimb.2022.916702. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35909975 Free PMC article. Review.
-
Autochthonous simian malaria in Brazil outside the Amazon: Emergence, zoonotic transmission and implications for disease control.One Health. 2024 Nov 19;19:100928. doi: 10.1016/j.onehlt.2024.100928. eCollection 2024 Dec. One Health. 2024. PMID: 39650148 Free PMC article. Review.
Cited by
-
Complexity of malaria transmission dynamics in the Brazilian Atlantic Forest.Curr Res Parasitol Vector Borne Dis. 2021 May 31;1:100032. doi: 10.1016/j.crpvbd.2021.100032. eCollection 2021. Curr Res Parasitol Vector Borne Dis. 2021. PMID: 35284897 Free PMC article.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
-
Zoonotic Malaria Risk in Serra Do Mar, Atlantic Forest, Brazil.Microorganisms. 2023 Sep 30;11(10):2465. doi: 10.3390/microorganisms11102465. Microorganisms. 2023. PMID: 37894123 Free PMC article.
-
IgM antibody responses against Plasmodium antigens in neotropical primates in the Brazilian Atlantic Forest.Front Cell Infect Microbiol. 2023 Sep 27;13:1169552. doi: 10.3389/fcimb.2023.1169552. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37829607 Free PMC article.
-
A machine learning framework to identify complex physicochemical features of B cell epitopes.Res Sq [Preprint]. 2025 Apr 18:rs.3.rs-6255613. doi: 10.21203/rs.3.rs-6255613/v1. Res Sq. 2025. PMID: 40321766 Free PMC article. Preprint.
References
-
- Abreu F. V. S., Santos E. D., Mello A. R. L., Gomes L. R., Alvarenga D. A. M., Gomes M. Q., et al. . (2019). Howler Monkeys are the Reservoir of Malarial Parasites Causing Zoonotic Infections in the Atlantic Forest of Rio De Janeiro. PloS Negl. Trop. Dis. 13 (12), e0007906. 10.1371/journal.pntd.0007906 - DOI - PMC - PubMed
-
- Barnwell J. E. (1986). Antigens of Plasmodium Vivax Blood Stage Parasites Identified by Monoclonal Antibodies. Mem Inst Oswaldo Cruz. 81 (Suppl 2), 56–61. 10.1590/S0074-02761986000600010 - DOI
-
- Brasil P., Zalis M. G., de Pina-Costa A., Siqueira A. M., Júnior C. B., Silva S., et al. . (2017). Outbreak of Human Malaria Caused by Plasmodium Simium in the Atlantic Forest in Rio De Janeiro: A Molecular Epidemiological Investigation. Lancet Glob Health 5 (10), 1038–1046. 10.1016/S2214-109X(17)30333-9 - DOI - PubMed
-
- Burkot T. R., Graves P. M., Wirtz R. A., Brabin B. J., Battistutta D., Cattani J. A., et al. . (1989). Differential Antibody Responses to Plasmodium Falciparum and P. Vivax Circumsporozoite Proteins in a Human Population. J. Clin. Microbiol. 27 (6), 1346–1351. 10.1128/JCM.27.6.1346-1351.1989 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

