The Leukodystrophy Spectrum in Saudi Arabia: Epidemiological, Clinical, Radiological, and Genetic Data
- PMID: 34055681
- PMCID: PMC8155587
- DOI: 10.3389/fped.2021.633385
The Leukodystrophy Spectrum in Saudi Arabia: Epidemiological, Clinical, Radiological, and Genetic Data
Abstract
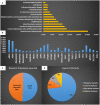
Background: Leukodystrophies (LDs) are inherited heterogeneous conditions that affect the central nervous system with or without peripheral nerve involvement. They are individually rare, but collectively, they are common. Thirty disorders were included by the Global Leukodystrophy Initiative Consortium (GLIA) as LDs. Methods: We conducted a retrospective chart review of a consecutive series of patients diagnosed with different types of LD from four large tertiary referral centers in Riyadh, Saudi Arabia. Only those 30 disorders defined by GLIA as LDs were included. Results: In total, 83 children from 61 families were identified and recruited for this study. The male-to-female ratio was 1.5:1, and a consanguinity rate of 58.5% was observed. An estimated prevalence of 1:48,780 or 2.05/100,000 was observed based on the clinical cohort, whereas a minimum of 1:32,857 or 3.04/100,000 was observed based on the local genetic database. The central region of the country exhibited the highest prevalence of LDs (48.5%). The most common LD was metachromatic leukodystrophy (MLD), and it accounted for 25.3%. The most common disorder based on carrier frequency was AGS. Novel variants were discovered in 51% of the cases, but 49% possessed previously reported variants. Missense variants were high in number and accounted for 73% of all cases. Compared with other disorders, MLD due to saposin b deficiency was more common than expected, Pelizaeus-Merzbacher-like disease was more prevalent than Pelizaeus-Merzbacher disease, and X-linked adrenoleukodystrophy was less common than expected. The mortality rate among our patients with LD was 24%. Conclusion: To the best of our knowledge, this is the largest cohort of patients with LD from Saudi Arabia. We present epidemiological, clinical, radiological, and genetic data. Furthermore, we report 18 variants that have not been reported previously. These findings are of great clinical and molecular utility for diagnosing and managing patients with LD.
Keywords: Saudi Arabia; leukodystrophy; metachromatic leukodystrophy; neurometabolic; novel mutations.
Copyright © 2021 Alfadhel, Almuqbil, Al Mutairi, Umair, Almannai, Alghamdi, Althiyab, Albarakati, Bashiri, Alshuaibi, Ba-Armah, Saleh, Al-Asmari, Faqeih, Altuwaijri, Al-Rumayyan, Balwi, Ababneh, Alswaid, Eyaid, Almontashiri, Alhashem, Hundallah, Bertoli-Avella, Bauer, Beetz, Alrifai, Alfares and Tabarki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Kufs H, Lange-Cosack H, Suckow J. [Clinical observations, histopathology and hereditary pathology in a family with familial juvenile diffuse sclerosis (leukodystrophia cerebri hereditaria progressiva)]. Psychiatr Neurol Med Psychol (Leipz). (1954) 6:12–24. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

