3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications
- PMID: 34055761
- PMCID: PMC8158943
- DOI: 10.3389/fbioe.2021.664188
3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications
Abstract
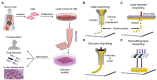
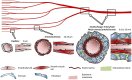
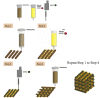
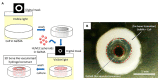
With a limited supply of organ donors and available organs for transplantation, the aim of tissue engineering with three-dimensional (3D) bioprinting technology is to construct fully functional and viable tissue and organ replacements for various clinical applications. 3D bioprinting allows for the customization of complex tissue architecture with numerous combinations of materials and printing methods to build different tissue types, and eventually fully functional replacement organs. The main challenge of maintaining 3D printed tissue viability is the inclusion of complex vascular networks for nutrient transport and waste disposal. Rapid development and discoveries in recent years have taken huge strides toward perfecting the incorporation of vascular networks in 3D printed tissue and organs. In this review, we will discuss the latest advancements in fabricating vascularized tissue and organs including novel strategies and materials, and their applications. Our discussion will begin with the exploration of printing vasculature, progress through the current statuses of bioprinting tissue/organoids from bone to muscles to organs, and conclude with relevant applications for in vitro models and drug testing. We will also explore and discuss the current limitations of vascularized tissue engineering and some of the promising future directions this technology may bring.
Keywords: 3D bioprinting; additive manufacturing; bioprinting; tissue engineering; vasculature.
Copyright © 2021 Chen, Toksoy, Davis and Geibel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Advances in tissue engineering of vasculature through three-dimensional bioprinting.Dev Dyn. 2021 Dec;250(12):1717-1738. doi: 10.1002/dvdy.385. Epub 2021 Jul 2. Dev Dyn. 2021. PMID: 34115420 Review.
-
Tissue Engineering Applications of Three-Dimensional Bioprinting.Cell Biochem Biophys. 2015 Jul;72(3):777-82. doi: 10.1007/s12013-015-0531-x. Cell Biochem Biophys. 2015. PMID: 25663505 Review.
-
Recent advances in 3D printing: vascular network for tissue and organ regeneration.Transl Res. 2019 Sep;211:46-63. doi: 10.1016/j.trsl.2019.04.002. Epub 2019 Apr 5. Transl Res. 2019. PMID: 31004563 Free PMC article. Review.
-
3D Bioprinting for Vascularized Tissue Fabrication.Ann Biomed Eng. 2017 Jan;45(1):132-147. doi: 10.1007/s10439-016-1653-z. Epub 2016 May 26. Ann Biomed Eng. 2017. PMID: 27230253 Free PMC article. Review.
-
Organ Bioprinting: Are We There Yet?Adv Healthc Mater. 2018 Jan;7(1). doi: 10.1002/adhm.201701018. Epub 2017 Nov 29. Adv Healthc Mater. 2018. PMID: 29193879 Review.
Cited by
-
3D Bioprinting for Pancreas Engineering/Manufacturing.Polymers (Basel). 2022 Nov 25;14(23):5143. doi: 10.3390/polym14235143. Polymers (Basel). 2022. PMID: 36501537 Free PMC article. Review.
-
Blood vessels in a dish: the evolution, challenges, and potential of vascularized tissues and organoids.Front Cardiovasc Med. 2024 Jun 13;11:1336910. doi: 10.3389/fcvm.2024.1336910. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38938652 Free PMC article. Review.
-
helixCAM: A platform for programmable cellular assembly in bacteria and human cells.Cell. 2022 Sep 15;185(19):3551-3567.e39. doi: 10.1016/j.cell.2022.08.012. Epub 2022 Sep 1. Cell. 2022. PMID: 36055250 Free PMC article.
-
The Effects of Three Chlorhexidine-Based Mouthwashes on Human Osteoblast-Like SaOS-2 Cells. An In Vitro Study.Int J Mol Sci. 2021 Sep 15;22(18):9986. doi: 10.3390/ijms22189986. Int J Mol Sci. 2021. PMID: 34576150 Free PMC article.
-
Revolutionizing Disease Modeling: The Emergence of Organoids in Cellular Systems.Cells. 2023 Mar 18;12(6):930. doi: 10.3390/cells12060930. Cells. 2023. PMID: 36980271 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

