Ecm29-Dependent Proteasome Localization Regulates Cytoskeleton Remodeling at the Immune Synapse
- PMID: 34055780
- PMCID: PMC8155528
- DOI: 10.3389/fcell.2021.650817
Ecm29-Dependent Proteasome Localization Regulates Cytoskeleton Remodeling at the Immune Synapse
Abstract
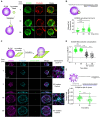
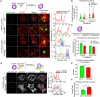
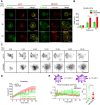
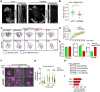
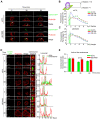
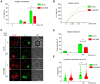
The formation of an immune synapse (IS) enables B cells to capture membrane-tethered antigens, where cortical actin cytoskeleton remodeling regulates cell spreading and depletion of F-actin at the centrosome promotes the recruitment of lysosomes to facilitate antigen extraction. How B cells regulate both pools of actin, remains poorly understood. We report here that decreased F-actin at the centrosome and IS relies on the distribution of the proteasome, regulated by Ecm29. Silencing Ecm29 decreases the proteasome pool associated to the centrosome of B cells and shifts its accumulation to the cell cortex and IS. Accordingly, Ecm29-silenced B cells display increased F-actin at the centrosome, impaired centrosome and lysosome repositioning to the IS and defective antigen extraction and presentation. Ecm29-silenced B cells, which accumulate higher levels of proteasome at the cell cortex, display decreased actin retrograde flow in lamellipodia and enhanced spreading responses. Our findings support a model where B the asymmetric distribution of the proteasome, mediated by Ecm29, coordinates actin dynamics at the centrosome and the IS, promoting lysosome recruitment and cell spreading.
Keywords: B cell; Ecm29; actin cytoskeleton; immune synapse; lysosomes; microtubules; proteasome.
Copyright © 2021 Ibañez-Vega, Del Valle, Sáez, Guzman, Diaz, Soza and Yuseff.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Lymphocyte Polarization During Immune Synapse Assembly: Centrosomal Actin Joins the Game.Front Immunol. 2022 Feb 11;13:830835. doi: 10.3389/fimmu.2022.830835. eCollection 2022. Front Immunol. 2022. PMID: 35222415 Free PMC article. Review.
-
Proteasome Dependent Actin Remodeling Facilitates Antigen Extraction at the Immune Synapse of B Cells.Front Immunol. 2019 Feb 19;10:225. doi: 10.3389/fimmu.2019.00225. eCollection 2019. Front Immunol. 2019. PMID: 30873155 Free PMC article.
-
The exocyst controls lysosome secretion and antigen extraction at the immune synapse of B cells.J Cell Biol. 2019 Jul 1;218(7):2247-2264. doi: 10.1083/jcb.201811131. Epub 2019 Jun 13. J Cell Biol. 2019. PMID: 31197029 Free PMC article.
-
Studying Organelle Dynamics in B Cells During Immune Synapse Formation.J Vis Exp. 2019 Jun 1;(148). doi: 10.3791/59621. J Vis Exp. 2019. PMID: 31205311
-
Role of Actin Cytoskeleton Reorganization in Polarized Secretory Traffic at the Immunological Synapse.Front Cell Dev Biol. 2021 Feb 4;9:629097. doi: 10.3389/fcell.2021.629097. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33614660 Free PMC article. Review.
Cited by
-
Lymphocyte Polarization During Immune Synapse Assembly: Centrosomal Actin Joins the Game.Front Immunol. 2022 Feb 11;13:830835. doi: 10.3389/fimmu.2022.830835. eCollection 2022. Front Immunol. 2022. PMID: 35222415 Free PMC article. Review.
-
Localized Proteasomal Degradation: From the Nucleus to Cell Periphery.Biomolecules. 2022 Jan 29;12(2):229. doi: 10.3390/biom12020229. Biomolecules. 2022. PMID: 35204730 Free PMC article. Review.
-
The Ins and Outs of Antigen Uptake in B cells.Front Immunol. 2022 Apr 26;13:892169. doi: 10.3389/fimmu.2022.892169. eCollection 2022. Front Immunol. 2022. PMID: 35572544 Free PMC article. Review.
-
B cell mechanosensing regulates ER remodeling at the immune synapse.Front Immunol. 2024 Oct 7;15:1464000. doi: 10.3389/fimmu.2024.1464000. eCollection 2024. Front Immunol. 2024. PMID: 39434873 Free PMC article.
-
Centrosomal enrichment and proteasomal degradation of SYS-1/β-catenin requires the microtubule motor dynein.Mol Biol Cell. 2022 May 1;33(5):ar42. doi: 10.1091/mbc.E22-02-0031. Epub 2022 Feb 23. Mol Biol Cell. 2022. PMID: 35196020 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

