Mesenchymal Epithelial Transition Factor Signaling in Pediatric Nervous System Tumors: Implications for Malignancy and Cancer Stem Cell Enrichment
- PMID: 34055785
- PMCID: PMC8155369
- DOI: 10.3389/fcell.2021.654103
Mesenchymal Epithelial Transition Factor Signaling in Pediatric Nervous System Tumors: Implications for Malignancy and Cancer Stem Cell Enrichment
Abstract
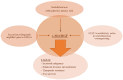
Malignant nervous system cancers in children are the most devastating and worrisome diseases, specifically due to their aggressive nature and, in some cases, inoperable location in critical regions of the brain and spinal cord, and the impermeable blood-brain barrier that hinders delivery of pharmaco-therapeutic compounds into the tumor site. Moreover, the delicate developmental processes of the nervous system throughout the childhood years adds another limitation to the therapeutic modalities and doses used to treat these malignant cancers. Therefore, pediatric oncologists are charged with the daunting responsibility of attempting to deliver effective cures to these children, yet with limited doses of the currently available therapeutic options in order to mitigate the imminent neurotoxicity of radio- and chemotherapy on the developing nervous system. Various studies reported that c-Met/HGF signaling is affiliated with increased malignancy and stem cell enrichment in various cancers such as high-grade gliomas, high-risk medulloblastomas, and MYCN-amplified, high-risk neuroblastomas. Therapeutic interventions that are utilized to target c-Met signaling in these malignant nervous system cancers have shown benefits in basic translational studies and preclinical trials, but failed to yield significant clinical benefits in patients. While numerous pre-clinical data reported promising results with the use of combinatorial therapy that targets c-Met with other tumorigenic pathways, therapeutic resistance remains a problem, and long-term cures are rare. The possible mechanisms, including the overexpression and activation of compensatory tumorigenic mechanisms within the tumors or ineffective drug delivery methods that may contribute to therapeutic resistance observed in clinical trials are elaborated in this review.
Keywords: cancer stem cells; hepatocyte growth factor/scatter factor; mesenchymal epithelial transition factor signaling; pediatric nervous system tumors; therapeutic resistance.
Copyright © 2021 Khater and Abou-Antoun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The hepatocyte growth factor/mesenchymal epithelial transition factor axis in high-risk pediatric solid tumors and the anti-tumor activity of targeted therapeutic agents.Front Pediatr. 2022 Aug 10;10:910268. doi: 10.3389/fped.2022.910268. eCollection 2022. Front Pediatr. 2022. PMID: 36034555 Free PMC article. Review.
-
Expression of hepatocyte growth factor/scatter factor and its receptor c-Met in brain tumors: evidence for a role in progression of astrocytic tumors (Review).Int J Mol Med. 1999 May;3(5):531-6. doi: 10.3892/ijmm.3.5.531. Int J Mol Med. 1999. PMID: 10202187 Review.
-
Role of HGF/MET axis in resistance of lung cancer to contemporary management.Transl Lung Cancer Res. 2012 Sep;1(3):179-93. doi: 10.3978/j.issn.2218-6751.2012.09.04. Transl Lung Cancer Res. 2012. PMID: 25806180 Free PMC article. Review.
-
Aberrant signaling pathways in medulloblastomas: a stem cell connection.Arq Neuropsiquiatr. 2010 Dec;68(6):947-52. doi: 10.1590/s0004-282x2010000600021. Arq Neuropsiquiatr. 2010. PMID: 21243257 Review.
-
Status of Agents Targeting the HGF/c-Met Axis in Lung Cancer.Cancers (Basel). 2018 Aug 21;10(9):280. doi: 10.3390/cancers10090280. Cancers (Basel). 2018. PMID: 30134579 Free PMC article. Review.
Cited by
-
Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met.Pharmaceutics. 2023 Jul 10;15(7):1915. doi: 10.3390/pharmaceutics15071915. Pharmaceutics. 2023. PMID: 37514101 Free PMC article.
-
IFITM3 promotes malignant progression, cancer stemness and chemoresistance of gastric cancer by targeting MET/AKT/FOXO3/c-MYC axis.Cell Biosci. 2022 Aug 8;12(1):124. doi: 10.1186/s13578-022-00858-8. Cell Biosci. 2022. PMID: 35941699 Free PMC article.
-
Tumor matrix stiffness provides fertile soil for cancer stem cells.Cancer Cell Int. 2023 Jul 20;23(1):143. doi: 10.1186/s12935-023-02992-w. Cancer Cell Int. 2023. PMID: 37468874 Free PMC article. Review.
-
Identifying health research in the era of COVID-19: A scoping review.SAGE Open Med. 2023 Jun 8;11:20503121231180030. doi: 10.1177/20503121231180030. eCollection 2023. SAGE Open Med. 2023. PMID: 37324118 Free PMC article.
-
Cancer research in Lebanon: Scope of the most recent publications of an academic institution (Review).Oncol Lett. 2024 Jun 3;28(2):350. doi: 10.3892/ol.2024.14484. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38872861 Free PMC article. Review.
References
-
- Abou-Antoun T. J., Nazarian J., Ghanem A., Vukmanovic S., Sandler A. D. (2018). Molecular and functional analysis of anchorage independent, treatment-evasive neuroblastoma tumorspheres with enhanced malignant properties: a possible explanation for radio-therapy resistance. PLoS One 13:e0189711. 10.1371/journal.pone.0189711, PMID: - DOI - PMC - PubMed
-
- Angevin E., Spitaleri G., Rodon J., Dotti K., Isambert N., Salvagni S., et al. . (2017). A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur. J. Cancer 87, 131–139. 10.1016/j.ejca.2017.10.016, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

