Length of the Neurogenic Period-A Key Determinant for the Generation of Upper-Layer Neurons During Neocortex Development and Evolution
- PMID: 34055808
- PMCID: PMC8155536
- DOI: 10.3389/fcell.2021.676911
Length of the Neurogenic Period-A Key Determinant for the Generation of Upper-Layer Neurons During Neocortex Development and Evolution
Abstract
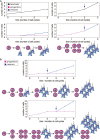
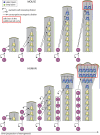
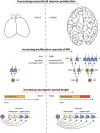
The neocortex, a six-layer neuronal brain structure that arose during the evolution of, and is unique to, mammals, is the seat of higher order brain functions responsible for human cognitive abilities. Despite its recent evolutionary origin, it shows a striking variability in size and folding complexity even among closely related mammalian species. In most mammals, cortical neurogenesis occurs prenatally, and its length correlates with the length of gestation. The evolutionary expansion of the neocortex, notably in human, is associated with an increase in the number of neurons, particularly within its upper layers. Various mechanisms have been proposed and investigated to explain the evolutionary enlargement of the human neocortex, focussing in particular on changes pertaining to neural progenitor types and their division modes, driven in part by the emergence of human-specific genes with novel functions. These led to an amplification of the progenitor pool size, which affects the rate and timing of neuron production. In addition, in early theoretical studies, another mechanism of neocortex expansion was proposed-the lengthening of the neurogenic period. A critical role of neurogenic period length in determining neocortical neuron number was subsequently supported by mathematical modeling studies. Recently, we have provided experimental evidence in rodents directly supporting the mechanism of extending neurogenesis to specifically increase the number of upper-layer cortical neurons. Moreover, our study examined the relationship between cortical neurogenesis and gestation, linking the extension of the neurogenic period to the maternal environment. As the exact nature of factors promoting neurogenic period prolongation, as well as the generalization of this mechanism for evolutionary distinct lineages, remain elusive, the directions for future studies are outlined and discussed.
Keywords: evolution; gestation; neocortex; neurogenic period length; upper-layer neurons.
Copyright © 2021 Stepien, Vaid and Huttner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Allman J. M. (1999). Evolving Brains. New York, NY: Scientifc American Library.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

