Low Protein Diets and Energy Balance: Mechanisms of Action on Energy Intake and Expenditure
- PMID: 34055853
- PMCID: PMC8155302
- DOI: 10.3389/fnut.2021.655833
Low Protein Diets and Energy Balance: Mechanisms of Action on Energy Intake and Expenditure
Abstract
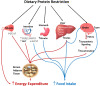
Low protein diets are associated with increased lifespan and improved cardiometabolic health primarily in rodents, and likely improve human health. There is strong evidence that moderate to severe reduction in dietary protein content markedly influences caloric intake and energy expenditure, which is often followed by a decrease in body weight and adiposity in animal models. While the neuroendocrine signals that trigger hyperphagic responses to protein restriction are better understood, there is accumulating evidence that increased sympathetic flux to brown adipose tissue, fibroblast growth factor-21 and serotonergic signaling are important for the thermogenic effects of low protein diets. This mini-review specifically focuses on the effect of low protein diets with variable carbohydrate and lipid content on energy intake and expenditure, and the underlying mechanisms of actions by these diets. Understanding the mechanisms by which protein restriction influences energy balance may unveil novel approaches for treating metabolic disorders in humans and improve production efficiency in domestic animals.
Keywords: energy balance; energy expenditure; food intake; low protein; neuroendocrine.
Copyright © 2021 Pezeshki and Chelikani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

