A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine
- PMID: 34056055
- PMCID: PMC8149168
- DOI: 10.1016/j.jdcr.2021.05.010
A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine
Keywords: AGEP; AGEP, acute generalized exanthematous pustulosis; COVID-19; DRESS; DRESS, drug reaction with eosinophilia and systemic symptoms; SCAR, severe cutaneous adverse reaction; severe adverse cutaneous reaction; vaccine.
Conflict of interest statement
None declared.
Figures
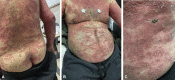

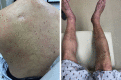
Comment in
-
Overlapping acute generalized exanthematous pustulosis drug reaction with eosinophilia and systemic symptoms induced by a second dose of the Moderna COVID-19 vaccine.J Dermatol. 2022 Dec;49(12):e446-e447. doi: 10.1111/1346-8138.16541. Epub 2022 Jul 29. J Dermatol. 2022. PMID: 35906789 Free PMC article. No abstract available.
References
-
- Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021;325(15):1575. - PubMed
-
- Wadman M. Public needs to prep for vaccine side effects. Science. 2020;370(6520):1022. - PubMed
-
- Jedlowski P.M., Jedlowski M.F. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol Online J. 2021;27(1) 13030/qt4xs486zg. - PubMed
-
- Duong T.A., Valeyrie-Allanore L., Wolkenstein P., Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106):1996–2011. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
