A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development
- PMID: 34056083
- PMCID: PMC8029445
- DOI: 10.1021/acscentsci.1c00120
A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development
Abstract
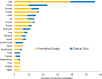
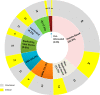
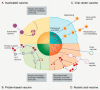
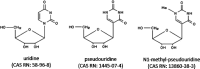
This report examines various vaccine platforms including inactivated vaccines, protein-based vaccines, viral vector vaccines, and nucleic acid (DNA or mRNA) vaccines, and their ways of producing immunogens in cells.
Published 2021 by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


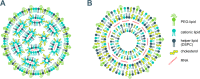

References
-
- COVID-19 Update: FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-... (accessed March 1, 2021).
-
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... (accessed March 1, 2021).
-
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... (accessed March 1, 2021).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

