Borrowing Hydrogen for Organic Synthesis
- PMID: 34056087
- PMCID: PMC8155478
- DOI: 10.1021/acscentsci.1c00125
Borrowing Hydrogen for Organic Synthesis
Abstract
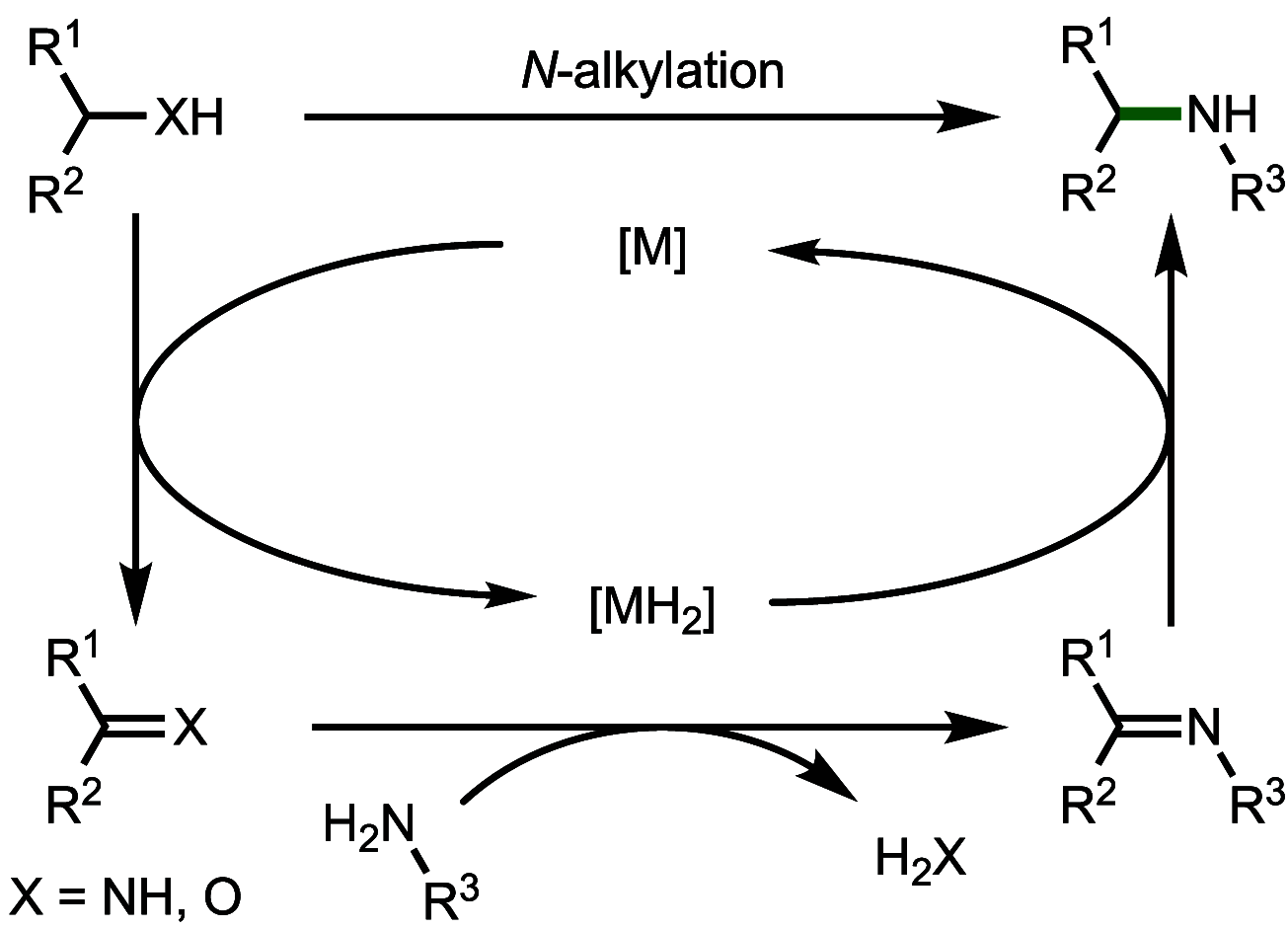
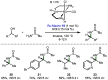
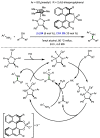
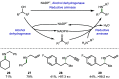
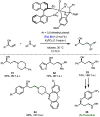
Borrowing hydrogen is a process that is used to diversify the synthetic utility of commodity alcohols. A catalyst first oxidizes an alcohol by removing hydrogen to form a reactive carbonyl compound. This intermediate can undergo a diverse range of subsequent transformations before the catalyst returns the "borrowed" hydrogen to liberate the product and regenerate the catalyst. In this way, alcohols may be used as alkylating agents whereby the sole byproduct of this one-pot reaction is water. In recent decades, significant advances have been made in this area, demonstrating many effective methods to access valuable products. This outlook highlights the diversity of metal and biocatalysts that are available for this approach, as well as the various transformations that can be performed, focusing on a selection of the most significant and recent advances. By succinctly describing and conveying the versatility of borrowing hydrogen chemistry, we anticipate its uptake will increase across a wider scientific audience, expanding opportunities for further development.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

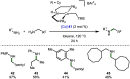

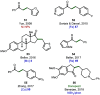
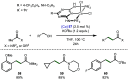
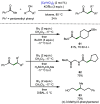

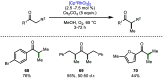
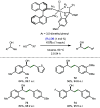
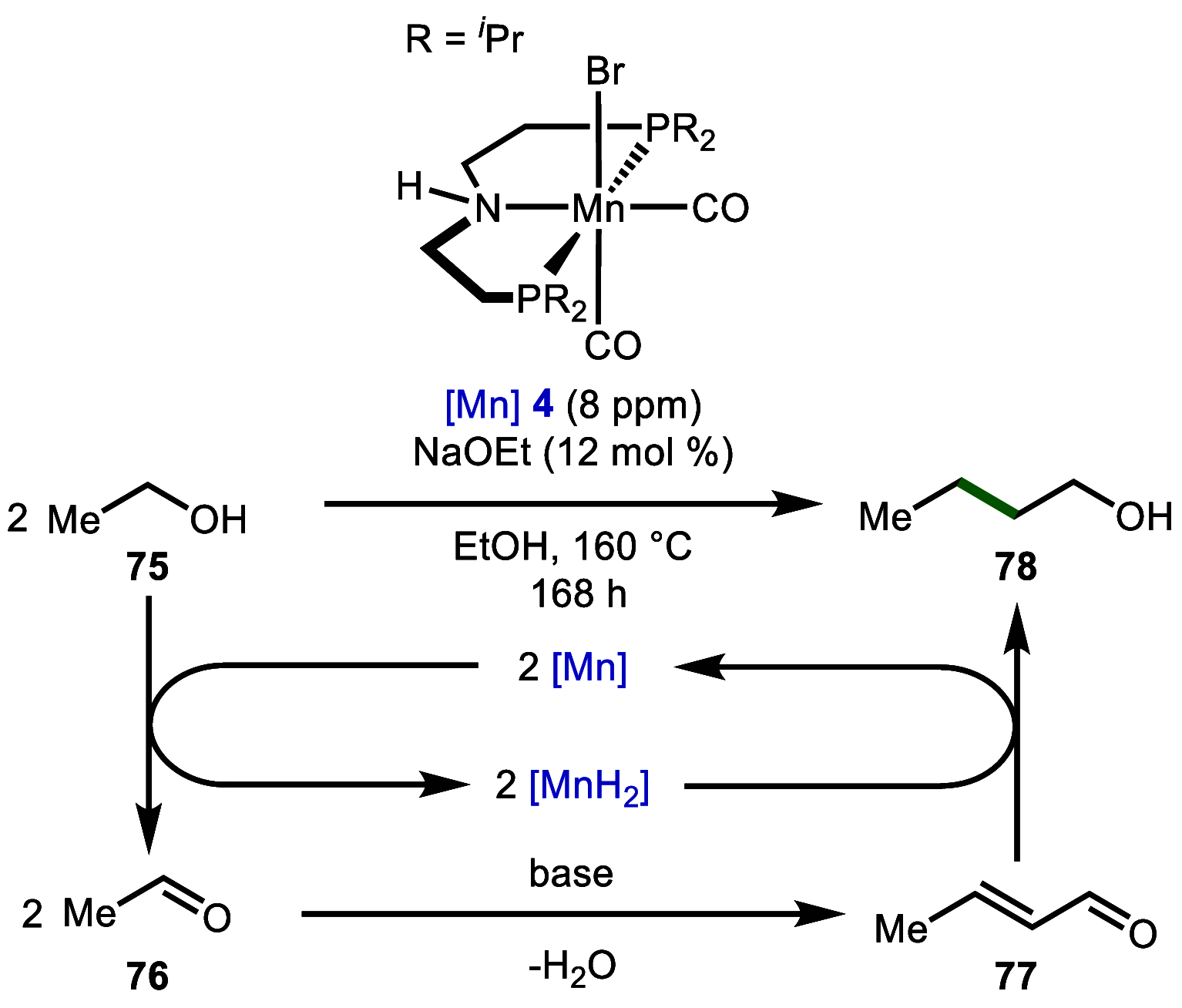

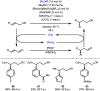

References
-
- Andersson P. G., Munslow I. J., Eds.; Modern Reduction Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2008;10.1002/9783527622115. - DOI
-
- Freeman I. P.; Melnikov S. M.. Margarines. Ullmann’s Encyclopedia of Industrial Chemistry; 2015; pp 1–24,10.1002/14356007.a16_145.pub2. - DOI
-
- Bonrath W.; Medlock J.; Schütz J.; Wüstenberg B.; Netscher T.. Hydrogenation in the Vitamins and Fine Chemicals Industry – An Overview. In Hydrogenation; Karamé I., Ed.; IntechOpen: 2012; pp 69–90,10.5772/48751. - DOI
-
- Shimizu K. I. Heterogeneous Catalysis for the Direct Synthesis of Chemicals by Borrowing Hydrogen Methodology. Catal. Sci. Technol. 2015, 5 (3), 1412–1427. 10.1039/C4CY01170H. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

