Pulmonary stromal expansion and intra-alveolar coagulation are primary causes of COVID-19 death
- PMID: 34056141
- PMCID: PMC8141733
- DOI: 10.1016/j.heliyon.2021.e07134
Pulmonary stromal expansion and intra-alveolar coagulation are primary causes of COVID-19 death
Abstract
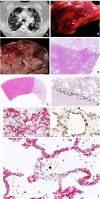
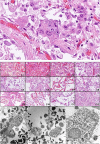
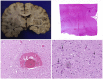
Most COVID-19 victims are old and die from unrelated causes. Here we present twelve complete autopsies, including two rapid autopsies of young patients where the cause of death was COVID-19 ARDS. The main virus induced pathology was in the lung parenchyma and not in the airways. Most coagulation events occurred in the intra-alveolar and not in the intra-vascular space and the few thrombi were mainly composed of aggregated thrombocytes. The dominant inflammatory response was the massive accumulation of CD163 + macrophages and the disappearance of T killer, NK and B-cells. The virus was replicating in the pneumocytes and macrophages but not in bronchial epithelium, endothelium, pericytes or stromal cells. The lung consolidations were produced by a massive regenerative response, stromal and epithelial proliferation and neovascularization. We suggest that thrombocyte aggregation inhibition, angiogenesis inhibition and general proliferation inhibition may have a roll in the treatment of advanced COVID-19 ARDS.
Keywords: ARDS; Autopsy; COVID-19.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

