Simple Fluorogenic Cellular Assay for Histone Deacetylase Inhibitors Based on Split-Yellow Fluorescent Protein and Intrabodies
- PMID: 34056159
- PMCID: PMC8153662
- DOI: 10.1021/acsomega.0c06281
Simple Fluorogenic Cellular Assay for Histone Deacetylase Inhibitors Based on Split-Yellow Fluorescent Protein and Intrabodies
Abstract
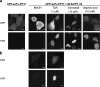
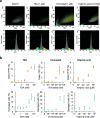
Histone deacetylase (HDAC) inhibitors that regulate the posttranslational modifications of histone tails are therapeutic drugs for many diseases such as cancers, neurodegenerative diseases, and asthma; however, convenient and sensitive methods to measure the effect of HDAC inhibitors in cultured mammalian cells remain limited. In this study, a fluorogenic assay was developed to detect the acetylation of lysine 9 on histone H3 (H3K9ac), which is involved in several cancers, Alzheimer's disease, and autism spectrum disorder. To monitor the changes in H3K9ac levels, an H3K9ac-specific intrabody fused with a small fragment FP11 of the split-yellow fluorescent protein (YFP) (scFv-FP11) was expressed in mammalian cells, together with a larger YFP fragment FP1-10 fused with a nuclear localization signal. When the intranuclear level of H3K9ac is increased, the scFv-FP11 is more enriched in the nucleus via passive diffusion through the nuclear pores from the cytoplasm, which increases the chance of forming a fluorescent complex with the nuclear YFP1-10. The results showed that the YFP fluorescence increased when the cells were treated with HDAC inhibitors. Moreover, the sensitivity of the split YFP reporter system to three HDAC inhibitors was higher than that of a conventional cell viability test. The assay system will be a simple and sensitive detection method to evaluate HDAC inhibitor activities at the levels of both single cells and cell populations.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Chen Z.; Li W.; Qiu F.; Huang Q.; Jiang Z.; Ye J.; Cheng P.; Low C.; Guo Y.; Yi X.; Chen W.; Yu Y.; Han Y.; Wu J.; Jin S.; Kong D.; Huang J. Aspirin cooperates with p300 to activate the acetylation of H3K9 and promote FasL-mediated apoptosis of cancer stem-like cells in colorectal cancer. Theranostics 2018, 8, 4447–4461. 10.7150/thno.24284. - DOI - PMC - PubMed
-
- Gao X.; Lin S. H.; Ren F.; Li J. T.; Chen J. J.; Yao C. B.; Yang H. B.; Jiang S. X.; Yan G. Q.; Wang D.; Wang Y.; Liu Y.; Cai Z.; Xu Y. Y.; Chen J.; Yu W.; Yang P. Y.; Lei Q. Y. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 2016, 7, 1196010.1038/ncomms11960. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

