Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine
- PMID: 34056192
- PMCID: PMC8153789
- DOI: 10.1021/acsomega.1c00772
Progress Toward a Large-Scale Synthesis of Molnupiravir (MK-4482, EIDD-2801) from Cytidine
Abstract
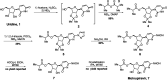
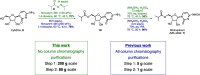
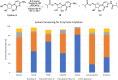
Molnupiravir (MK-4482, EIDD-2801) is a promising orally bioavailable drug candidate for the treatment of COVID-19. Herein, we describe a supply-centered and chromatography-free synthesis of molnupiravir from cytidine, consisting of two steps: a selective enzymatic acylation followed by transamination to yield the final drug product. Both steps have been successfully performed on a decagram scale: the first step at 200 g and the second step at 80 g. Overall, molnupiravir has been obtained in a 41% overall isolated yield compared to a maximum 17% isolated yield in the patented route. This route provides many advantages to the initial route described in the patent literature and would decrease the cost of this pharmaceutical should it prove safe and efficacious in ongoing clinical trials.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
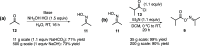
References
-
- Cross R. Merck & Co. Joins Race for COVID-19 Vaccines and Therapies. Chem. Eng. News 2020, 98, 12.10.47287/cen-09844-buscon1. - DOI
-
- Sheahan T. P.; Sims A. C.; Zhou S.; Graham R. L.; Pruijssers A. J.; Agostini M. L.; Leist S. R.; Schäfer A.; Dinnon K. H.; Stevens L. J.; Chappell J. D.; Lu X.; Hughes T. M.; George A. S.; Hill C. S.; Montgomery S. A.; Brown A. J.; Bluemling G. R.; Natchus M. G.; Saindane M.; Kolykhalov A. A.; Painter G.; Harcourt J.; Tamin A.; Thornburg N. J.; Swanstrom R.; Denison M. R.; Baric R. S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb588310.1126/scitranslmed.abb5883. - DOI - PMC - PubMed
-
- Painter G. R.; Bluemling G. R.; Natchus M. G.; Guthrie D.. N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto. WO2019113462A12019
LinkOut - more resources
Full Text Sources
Other Literature Sources

