Toxicological Screening of 4-Phenyl-3,4-dihydrobenzo[ h]quinolin-2(1 H)-one: A New Potential Candidate for Alzheimer's Treatment
- PMID: 34056243
- PMCID: PMC8153932
- DOI: 10.1021/acsomega.1c00654
Toxicological Screening of 4-Phenyl-3,4-dihydrobenzo[ h]quinolin-2(1 H)-one: A New Potential Candidate for Alzheimer's Treatment
Retraction in
-
Retraction of "Toxicological Screening of 4-Phenyl-3,4-dihydrobenzo[h]quinolin-2(1H)-one: A New Potential Candidate for Alzheimer's Treatment".ACS Omega. 2024 Sep 6;9(37):39296. doi: 10.1021/acsomega.4c08066. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310159 Free PMC article.
Abstract
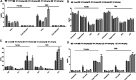
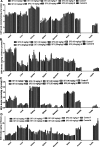
Toxicity studies are necessary for the development of a new drug. Naphthalene is a bicyclic molecule and is easy to derivatize. In our previous study, a derivative of naphthalene (4-phenyl,3,4-dihydrobenzoquinoline-2(H)one) was synthesized and reported its in vitro activity on different enzymes. This study was a probe to investigate the toxicity potential of that compound (SF3). Acute oral (425), subacute (407), and teratogenicity (414) studies were planned according to their respective guidelines given by organization of economic cooperation and development (OECD). Acute oral, subacute, and teratogenicity studies were carried out on 2000, 5-40, and 40 mg/kg doses. Blood samples were collected for hematological and biochemical analyses. Vital organs were excised for oxidative stress (superoxide dismutase, catalase, glutathione, and malondialdehyde) and histopathological analysis. LD 50 of SF3 was higher than 2000 mg/kg. In acute and subacute studies, levels of alkaline phosphates and aspartate transaminase were increased. Teratogenicity showed no resorptions, no skeletal or soft tissue abnormalities, and no cleft pallet. Oxidative stress biomarkers were close to the normal, and no increase in the malondialdehyde level was seen. Histopathological studies revealed normal tissue architecture of the selected organs, except kidney, in acute oral and subacute toxicity studies at 40 mg/kg. The study concluded that SF3 is safer if used as a drug.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Zhang H.-J.; Rumschlag-Booms E.; Guan Y.-F.; Wang D.-Y.; Liu K.-L.; Li W.-F.; Nguyen V. H.; Cuong N. M.; Soejarto D. D.; Fong H. H. S.; Rong L. Potent Inhibitor of Drug-Resistant HIV-1 Strains Identified from the Medicinal PlantJusticia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. 10.1021/acs.jnatprod.7b00004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

