Discovery of Novel CCR5 Ligands as Anticolorectal Cancer Agents by Sequential Virtual Screening
- PMID: 34056245
- PMCID: PMC8153923
- DOI: 10.1021/acsomega.1c00681
Discovery of Novel CCR5 Ligands as Anticolorectal Cancer Agents by Sequential Virtual Screening
Abstract
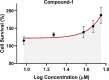
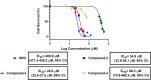
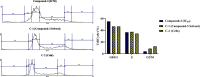
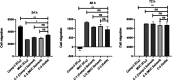
C-C chemokine receptor type 5 (CCR5) is a member of the G protein-coupled receptor. CCR5 and its interaction with chemokine ligands have been crucial for understanding and tackling human immunodeficiency virus (HIV)-1 entry into target cells. In recent years, the change in CCR5 expression has been related to the progression of different cancer types. Patients treated with the CCR5 ligand, maraviroc (MVC), showed a deceleration in tumor development especially for metastatic colorectal cancer. Based on the crystal structure of CCR5, we herein describe a multistage virtual screening protocol including pharmacophore screening, molecular docking, and protein-ligand interaction fingerprint (PLIF) postdocking filtration for discovery of novel CCR5 ligands. The applied virtual screening protocol led to the identification of four hits with binding modes showing access to the major and minor pockets of the MVC binding site. Compounds 2-4 showed a decrease in cellular proliferation upon testing on the metastatic colorectal cancer cell line, SW620, displaying 12, 16, and 4 times higher potency compared to MVC, respectively. Compound 3 induced apoptosis by arresting cells in the G0/G1 phase of the cell cycle similar to MVC. Further in vitro assays showed compound 3 drastically decreasing the CCR5 expression and cellular migration 48 h post treatment, indicating its ability to inhibit metastatic activity in SW620 cells. The discovered hits represent potential leads for the development of novel classes of anticolorectal cancer agents targeting CCR5.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Binding of fusion protein FLSC IgG1 to CCR5 is enhanced by CCR5 antagonist Maraviroc.Antiviral Res. 2014 Dec;112:80-90. doi: 10.1016/j.antiviral.2014.10.006. Epub 2014 Oct 24. Antiviral Res. 2014. PMID: 25453341 Free PMC article.
-
Impact of maraviroc-resistant and low-CCR5-adapted mutations induced by in vitro passage on sensitivity to anti-envelope neutralizing antibodies.J Gen Virol. 2014 Aug;95(Pt 8):1816-1826. doi: 10.1099/vir.0.062885-0. Epub 2014 May 2. J Gen Virol. 2014. PMID: 24795449
-
Synergistic Inhibition of R5 HIV-1 by the Fusion Protein (FLSC) IgG1 Fc and Maraviroc in Primary Cells: Implications for Prevention and Treatment.Curr HIV Res. 2016;14(1):24-36. doi: 10.2174/1570162x13666150909145150. Curr HIV Res. 2016. PMID: 26354735
-
CCR5 inhibitors: emergence, success, and challenges.Expert Opin Emerg Drugs. 2012 Jun;17(2):135-45. doi: 10.1517/14728214.2012.673584. Epub 2012 Apr 25. Expert Opin Emerg Drugs. 2012. PMID: 22533737 Review.
-
Clinical studies with chemokine receptor-5 (CCR5)-inhibitors.Curr Opin HIV AIDS. 2012 Sep;7(5):456-62. doi: 10.1097/COH.0b013e328356e933. Curr Opin HIV AIDS. 2012. PMID: 22832708 Review.
Cited by
-
Targeting Neutrophil-Mediated Inflammation: Identification of Pyrazolidinone Carboxamide Derivatives as Potent Selective Inhibitors of Formyl Peptide Receptor 1 (FPR1)-Activated Neutrophils.ACS Pharmacol Transl Sci. 2025 May 2;8(6):1591-1609. doi: 10.1021/acsptsci.4c00715. eCollection 2025 Jun 13. ACS Pharmacol Transl Sci. 2025. PMID: 40534665
-
Biochemical and biological studies of irradiated and non-irradiated extracts of Solanum aculeastrum Dunal fruit.Sci Rep. 2024 Oct 22;14(1):24829. doi: 10.1038/s41598-024-73531-4. Sci Rep. 2024. PMID: 39438506 Free PMC article.
-
Synthesis, Characterisation, Biological Evaluation and In Silico Studies of Quinoline-1,2,3-Triazole-Anilines as Potential Antitubercular and Anti-HIV Agents.Molecules. 2025 May 10;30(10):2119. doi: 10.3390/molecules30102119. Molecules. 2025. PMID: 40430292 Free PMC article.
-
Bridging Structure- and Ligand-Based Virtual Screening through Fragmented Interaction Fingerprint.ACS Omega. 2024 Sep 3;9(37):38957-38969. doi: 10.1021/acsomega.4c05433. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310180 Free PMC article.
-
Assessment of the RANTES Level Correlation and Selected Inflammatory and Pro-Angiogenic Molecules Evaluation of Their Influence on CRC Clinical Features: A Preliminary Observational Study.Medicina (Kaunas). 2022 Jan 28;58(2):203. doi: 10.3390/medicina58020203. Medicina (Kaunas). 2022. PMID: 35208526 Free PMC article.
References
-
- Bachelerie F.; Ben-Baruch A.; Burkhardt A. M.; Combadiere C.; Farber J. M.; Graham G. J.; Horuk R.; Sparre-Ulrich A. H.; Locati M.; Luster A. D.; Mantovani A.; Matsushima K.; Murphy P. M.; Nibbs R.; Nomiyama H.; Power C. A.; Proudfoot A. E. I.; Rosenkilde M. M.; Rot A.; Sozzani S.; Thelen M.; Yoshie O.; Zlotnik A. International Union of Pharmacology: LXXXIX: update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79. 10.1124/pr.113.007724. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

