Network Pharmacology Analysis and Experimental Pharmacology Study Explore the Mechanism of Gambogic Acid against Endometrial Cancer
- PMID: 34056247
- PMCID: PMC8153951
- DOI: 10.1021/acsomega.1c00696
Network Pharmacology Analysis and Experimental Pharmacology Study Explore the Mechanism of Gambogic Acid against Endometrial Cancer
Abstract
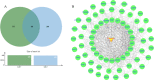
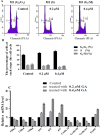
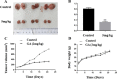
Endometrial cancer (EC) is one of the three most common gynecological cancers in female groups. Gambogic acid (GA), a natural caged xanthone, exerts significantly antitumor effects on many cancers. However, its efficacy on EC and pharmacological mechanism of action remain marginal up to now. This study suggested that GA had significant inhibitory effects on EC in vitro and in vivo, and no toxicity to normal cells or mice. In detail, GA suppressed cell proliferation, induced cell apoptosis, and cell cycle arrest at G0/G1 stage, complied with the network pharmacology analysis, showed that the PI3K/Akt pathways were the most important signaling, and their protein and mRNA expression levels were confirmed by qRT-PCR and Western blot experiments. In all, our study first proved that GA could inhibit cell proliferation, induce cell apoptosis, and cell cycle arrest at G0/G1 stage via the PI3K/Akt pathways, so GA would be a good therapy for EC.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor β tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells.J Atheroscler Thromb. 2010 Sep 30;17(9):901-13. doi: 10.5551/jat.3491. Epub 2010 Jun 11. J Atheroscler Thromb. 2010. PMID: 20543524
-
An Integrated Study on the Antitumor Effect and Mechanism of Triphala Against Gynecological Cancers Based on Network Pharmacological Prediction and In Vitro Experimental Validation.Integr Cancer Ther. 2018 Sep;17(3):894-901. doi: 10.1177/1534735418774410. Epub 2018 May 10. Integr Cancer Ther. 2018. PMID: 29742928 Free PMC article.
-
Combination of gambogic acid with cisplatin enhances the antitumor effects on cisplatin-resistant lung cancer cells by downregulating MRP2 and LRP expression.Onco Targets Ther. 2016 Jun 2;9:3359-68. doi: 10.2147/OTT.S100936. eCollection 2016. Onco Targets Ther. 2016. PMID: 27330316 Free PMC article.
-
Gambogic acid inhibits the growth of osteosarcoma cells in vitro by inducing apoptosis and cell cycle arrest.Oncol Rep. 2011 May;25(5):1289-95. doi: 10.3892/or.2011.1189. Epub 2011 Feb 17. Oncol Rep. 2011. PMID: 21331449
-
Emerging tendencies for the nano-delivery of gambogic acid: a promising approach in oncotherapy.RSC Adv. 2024 Feb 5;14(7):4666-4691. doi: 10.1039/d3ra08042k. eCollection 2024 Jan 31. RSC Adv. 2024. PMID: 38318629 Free PMC article. Review.
Cited by
-
Mechanism of anticancer effect of gambogic acid on gastric signet ring cell carcinoma.Med Oncol. 2023 Aug 16;40(9):269. doi: 10.1007/s12032-023-02149-9. Med Oncol. 2023. PMID: 37587317
-
Gambogic acid: Multi-gram scale isolation, stereochemical erosion toward epi-gambogic acid and biological profile.Front Nat Prod. 2022;1:1018765. doi: 10.3389/fntpr.2022.1018765. Front Nat Prod. 2022. PMID: 39211297 Free PMC article.
-
Study on the molecular mechanism of anti-liver cancer effect of Evodiae fructus by network pharmacology and QSAR model.Front Chem. 2023 Jan 9;10:1060500. doi: 10.3389/fchem.2022.1060500. eCollection 2022. Front Chem. 2023. PMID: 36700075 Free PMC article.
-
Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer.Antioxidants (Basel). 2023 Mar 16;12(3):735. doi: 10.3390/antiox12030735. Antioxidants (Basel). 2023. PMID: 36978983 Free PMC article. Review.
-
Caged Polyprenylated Xanthones in Garcinia hanburyi and the Biological Activities of Them.Drug Des Devel Ther. 2023 Dec 5;17:3625-3660. doi: 10.2147/DDDT.S426685. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38076632 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

