Distinct Modes of Tissue Expansion in Free Versus Earlier-Confined Boundaries for More Physiological Modeling of Wound Healing, Cancer Metastasis, and Tissue Formation
- PMID: 34056276
- PMCID: PMC8153934
- DOI: 10.1021/acsomega.0c06232
Distinct Modes of Tissue Expansion in Free Versus Earlier-Confined Boundaries for More Physiological Modeling of Wound Healing, Cancer Metastasis, and Tissue Formation
Abstract
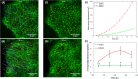
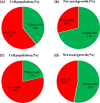
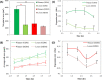
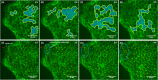
Collective cell migration is often seen in many biological processes like embryogenesis, cancer metastasis, and wound healing. Despite extensive experimental and theoretical research, the unified mechanism responsible for collective cell migration is not well known. Most of the studies have investigated artificial model wound to study the collective cell migration in an epithelial monolayer. These artificial model wounds possess a high cell number density compared to the physiological scenarios like wound healing (cell damage due to applied cut) and cancer metastasis (smaller cell clusters). Therefore, both systems may not completely relate to each other, and further investigation is needed to understand the collective cell migration in physiological scenarios. In an effort to fill this existing knowledge gap, we investigated the freely expanding monolayer that closely represented the physiological scenarios and compared it with the artificially created model wound. In the present work, we report the effect of initial boundary conditions (free and confined) on the collective cell migration of the epithelial cell monolayer. The expansion and migration aspects of the freely expanding and earlier-confined monolayer were investigated at the tissue and cellular levels. The freely expanding monolayer showed significantly higher expansion and lower migration in comparison to the earlier-confined monolayer. The expansion and migration rate of the monolayer exhibited a strong negative correlation. The study highlights the importance of initial boundary conditions in the collective cell migration of the expanding tissue and provides useful insights that might be helpful in the future to tune the collective cell migration in wound healing, cancer metastasis, and tissue formation.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Dynamic Migration Modes of Collective Cells.Biophys J. 2018 Nov 6;115(9):1826-1835. doi: 10.1016/j.bpj.2018.09.010. Epub 2018 Sep 20. Biophys J. 2018. PMID: 30297134 Free PMC article.
-
Computational Modeling of Collective Cell Migration: Mechanical and Biochemical Aspects.Adv Exp Med Biol. 2019;1146:1-11. doi: 10.1007/978-3-030-17593-1_1. Adv Exp Med Biol. 2019. PMID: 31612450 Review.
-
A Wound-Healing Assay Based on Ultraviolet Light Ablation.SLAS Technol. 2017 Feb;22(1):36-43. doi: 10.1177/2211068216646741. Epub 2016 Jul 10. SLAS Technol. 2017. PMID: 27139694
-
Collective Polarization of Cancer Cells at the Monolayer Boundary.Micromachines (Basel). 2021 Jan 22;12(2):112. doi: 10.3390/mi12020112. Micromachines (Basel). 2021. PMID: 33499191 Free PMC article.
-
Advances in wound-healing assays for probing collective cell migration.J Lab Autom. 2012 Feb;17(1):59-65. doi: 10.1177/2211068211426550. J Lab Autom. 2012. PMID: 22357609 Review.
Cited by
-
The Pertinent Role of Cell and Matrix Mechanics in Cell Adhesion and Migration.Front Cell Dev Biol. 2021 Oct 13;9:720494. doi: 10.3389/fcell.2021.720494. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34722504 Free PMC article. No abstract available.
-
The influence of viscosity of hydrogels on the spreading and migration of cells in 3D bioprinted skin cancer models.Front Cell Dev Biol. 2024 May 21;12:1391259. doi: 10.3389/fcell.2024.1391259. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38835508 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

