Printed Electrode for Measuring Phosphate in Environmental Water
- PMID: 34056285
- PMCID: PMC8153944
- DOI: 10.1021/acsomega.1c00132
Printed Electrode for Measuring Phosphate in Environmental Water
Abstract
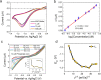
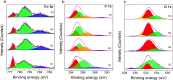
Phosphate is a major nonpoint source pollutant in both the Louisiana local streams as well as in the Gulf of Mexico coastal waters. Phosphates from agricultural run-off have contributed to the eutrophication of global surface waters. Phosphate environmental dissemination and eutrophication problems are not yet well understood. Thus, this study aimed to monitor phosphate in the local watershed to help identify potential hot spots in the local community (Mississippi River, Louisiana) that may contribute to nutrient loading downstream (in the Gulf of Mexico). An electrochemical method using a physical vapor deposited cobalt microelectrode was utilized for phosphate detection using cyclic voltammetry and amperometry. The testing results were utilized to evaluate the phosphate distribution in river water and characterize the performance of the microsensor. Various characterizations, including the limit of detection, sensitivity, and reliability, were conducted by measuring the effect of interferences, including dissolved oxygen, pH, and common ions. The electrochemical sensor performance was validated by comparing the results with the standard colorimetry phosphate detection method. X-ray photoelectron spectroscopy (XPS) measurements were performed to understand the phosphate sensing mechanism on the cobalt electrode. This proof-of-concept sensor chip could be utilized for on-field monitoring using a portable, hand-held potentiostat.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Haygarth P. M.; Jarvie H. P.; Powers S. M.; Sharpley A. N.; Elser J. J.; Shen J.; Peterson H. M.; Chan N.-I.; Howden N. J. K.; Burt T.; Worrall F.; Zhang F.; Liu X. Sustainable phosphorus management and the need for a long-term perspective: The legacy hypothesis. Environ. Sci. Technol. 2014, 48, 8417–8419. 10.1021/es502852s. - DOI - PubMed
-
- Vollenweider R. A.Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors in eutrophication. OECD Paris: 1970.
-
- Harris T. D.; Smith V. H.; Graham J. L.; Van de Waal D. B.; Tedesco L. P.; Clercin N. Combined effects of nitrogen to phosphorus and nitrate to ammonia ratios on cyanobacterial metabolite concentrations in eutrophic Midwestern USA reservoirs. Inland Waters 2016, 6, 199–210. 10.5268/IW-6.2.938. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

