Tuning Ni-MoO2 Catalyst-Ionomer and Electrolyte Interaction for Water Electrolyzers with Anion Exchange Membranes
- PMID: 34056552
- PMCID: PMC8159162
- DOI: 10.1021/acsaem.0c03072
Tuning Ni-MoO2 Catalyst-Ionomer and Electrolyte Interaction for Water Electrolyzers with Anion Exchange Membranes
Abstract
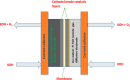
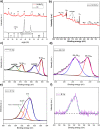
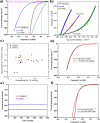
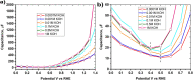
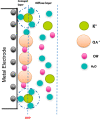
Tailoring catalyst-ionomer and electrolyte interaction is crucial for the development of anion exchange membrane (AEM) water electrolysis. In this work, the interaction of Ni-MoO2 nanosheets with ionomers and electrolyte cations was investigated. The activity of Ni-MoO2 nanosheets for the hydrogen evolution reaction (HER) increased when tested in 1 M NaOH compared to 1 M KOH; however, it decreased when tested in 0.01 M KOH compared to 1 M KOH electrolyte. The capacitance minimum associated with the potential of zero free charge (pzfc) was shifted negatively from 0.5 to 0.4 V versus RHE when KOH concentration increased from 0.1 mM to 1 M KOH, suggesting a softening of the water in the double-layer to facilitate the OH- transport and faster kinetics of the Volmer step that lead to improved HER activity. The catalyst interaction with cationic moieties in the anion ionomer (or organic electrolytes) can also be rationalized based on the capacitance minimum, because the latter indicates a negatively charged catalyst during the HER, attracting the cationic moieties leading to the blocking of the catalytic sites and lower HER performance. The HER activity of Ni-MoO2 nanosheets is lower in benzyltrimethylammonium hydroxide (BTMAOH) than in tetramethylammonium hydroxide (TMAOH). Anion fumion ionomer and electrolytes with organic cations with benzyl group adsorption (such as BTMAOH) lead to decreased HER activity in comparison with TMAOH and Nafion. By utilizing Ni-MoO2 nanosheet electrodes as a cathode in a full non-platinum group metal (PGM) AEM electrolyzer, a current density of 1.15 A/cm2 at 2 V cell voltage in 1 M KOH at 50 °C was achieved. The electrolyzer showed exceptional stability in 0.1 M KOH for 65 h at 0.5 A/cm2.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Abdalla A. M.; Hossain S.; Nisfindy O. B.; Azad A. T.; Dawood M.; Azad A. K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manage. 2018, 165 (March), 602–627. 10.1016/j.enconman.2018.03.088. - DOI
-
- Faid A.; Oyarce Barnett A.; Seland F.; Sunde S. Highly Active Nickel-Based Catalyst for Hydrogen Evolution in Anion Exchange Membrane Electrolysis. Catalysts 2018, 8 (12), 614. 10.3390/catal8120614. - DOI
-
- Faid A. Y.; Barnett A. O.; Seland F.; Sunde S. Optimized Nickel-Cobalt and Nickel-Iron Oxide Catalysts for the Hydrogen Evolution Reaction in Alkaline Water Electrolysis. J. Electrochem. Soc. 2019, 166 (8), F519–F533. 10.1149/2.0821908jes. - DOI
-
- Varcoe J. R.; Atanassov P.; Dekel D. R.; Herring A. M.; Hickner M. A.; Kohl P. A.; Kucernak A. R.; Mustain W. E.; Nijmeijer K.; Scott K.; Xu T.; Zhuang L. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7 (10), 3135–3191. 10.1039/C4EE01303D. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
