A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States
- PMID: 34056568
- PMCID: PMC8143650
- DOI: 10.1016/j.eclinm.2021.100902
A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States
Abstract
Background: This cross-sectional study aimed to track population-based SARS-CoV-2 antibody seropositivity duration across the United States using observational data from a national clinical laboratory registry of patients tested by nucleic acid amplification (NAAT) and serologic assays. Knowledge of antibody seropositivity and its duration may help dictate post-pandemic planning.
Methods: Using assays to detect antibodies to either nucleocapsid (N) or spike (S) proteins performed on specimens from 39,086 individuals with confirmed positive COVID-19 by reverse transcription-polymerase chain reaction (RT-PCR) from March 2020 to January 2021, we analyzed nationwide seropositivity rates of IgG up to 300 days following patients' initial positive NAAT test. Linear regression identified trends in seropositivity rates and logistic regression tested positive predictability by age, sex, assay type and days post-infection.
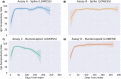
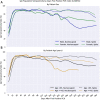
Findings: Seropositivity of IgG antibodies to both SARS-CoV-2 S and N-proteins followed a linear trend reaching approximately 90% positivity at 21 days post-index. The rate of N-protein seropositivity declined at a sharper rate, decaying to 68·2% [95% CI: 63·1-70·8%] after 293 days, while S-antibody seropositivity maintained a rate of 87·8% [95% CI: 86·3-89·1%] through 300 days. In addition to antigen type and the number of days post-positive PCR, age and gender were also significant factors in seropositivity prediction, with those under 65 years of age showing a more sustained seropositivity rate.
Interpretation: Observational data from a national clinical laboratory, though limited by an epidemiological view of the U.S. population, offer an encouraging timeline for the development and sustainability of antibodies up to ten months from natural infection and could inform post-pandemic planning.
Keywords: Antibody seropositivity; COVID-19; Real-world evidence; SARS-CoV-2.
© 2021 The Author(s).
Conflict of interest statement
None.
Figures


References
-
- Labcorp. LabCorp-COVID-19-antibody-tests-available-nationwide-no-upfront-out-of-pocket-costs [Internet]. 2020. Available from: https://www.labcorp.com/coronavirus-disease-covid-19/news/LabCorp-COVID-...
-
- Adams E., Ainsworth M., Anand R., Andersson R., Auckland K., Baillie J.K. Evaluation of antibody testing for SARS-CoV-2 using ELISA and lateral flow immunoassays. medRxiv. 2020
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

