Anti-viral treatment for SARS-CoV-2 infection: A race against time amidst the ongoing pandemic
- PMID: 34056571
- PMCID: PMC8143911
- DOI: 10.1016/j.metop.2021.100096
Anti-viral treatment for SARS-CoV-2 infection: A race against time amidst the ongoing pandemic
Abstract
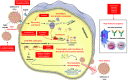
Remdesivir (GS-5734), a drug initially developed to treat hepatitis C and Ebola virus disease, was the first approved treatment for severe coronavirus disease 2019 (COVID-19). However, apart from remdesivir, there is a paucity of other specific anti-viral agents against SARS-CoV-2 infection. In 2017, researchers had documented the anti-coronavirus potential of remdesivir in animal models. At the same time, trials performed during two Ebola outbreaks in Africa showed that the drug was safe. Although vaccines against SARS-CoV-2 infection have emerged at an enormously high speed, equivalent results from efforts towards the development of anti-viral drugs, which could have played a truly life-saving role in the current stage of the pandemic, have been stagnating. In this review, we will focus on the current treatment options for COVID-19 which mainly consist of repurposed agents or treatments conferring passive immunity (convalescent plasma or monoclonal antibodies). Additionally, potential specific anti-viral therapies under development will be reviewed, such as the decoy miniprotein CTC-445.2d, protease inhibitors, mainly against the Main protein Mpro, nucleoside analogs, such as molnupiravir and compounds blocking the replication transcription complex proteins, such as zotatifin and plitidepsin. These anti-viral agents seem to be very promising but still require meticulous clinical trial testing in order to establish their efficacy and safety. The continuous emergence of viral variants may pose a real challenge to the scientific community towards that end. In this context, the advent of nanobodies together with the potential administration of a combination of anti-viral drugs could serve as useful tools in the armamentarium against COVID-19.
Keywords: Antiviral treatment; Baricitinib; COVID-19; Favipiravir; Molnupiravir; Monoclonal antibodies; Nanobodies; Plitidepsin; Protease inhibitors; Ribavirin; SARS-CoV-2; Zotatifin; boceprevir.
© 2021 Published by Elsevier Inc.
Conflict of interest statement
No conflict of interest to disclose.
Figures
References
-
- reportWHO Report. Coronavirus disease (COVID-19) pandemic. (Assessed on May 15, 2021).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

