A General Method to Improve Fluorophores Using Deuterated Auxochromes
- PMID: 34056637
- PMCID: PMC8154212
- DOI: 10.1021/jacsau.1c00006
A General Method to Improve Fluorophores Using Deuterated Auxochromes
Abstract
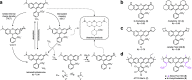
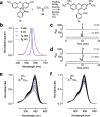
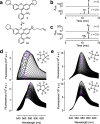
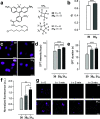
Fluorescence microscopy relies on dyes that absorb and then emit photons. In addition to fluorescence, fluorophores can undergo photochemical processes that decrease quantum yield or result in spectral shifts and irreversible photobleaching. Chemical strategies that suppress these undesirable pathways-thereby increasing the brightness and photostability of fluorophores-are crucial for advancing the frontier of bioimaging. Here, we describe a general method to improve small-molecule fluorophores by incorporating deuterium into the alkylamino auxochromes of rhodamines and other dyes. This strategy increases fluorescence quantum yield, inhibits photochemically induced spectral shifts, and slows irreparable photobleaching, yielding next-generation labels with improved performance in cellular imaging experiments.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Patents and patent applications describing azetidine- and deuterium-containing fluorophores (with inventors J.B.G. and L.D.L.) are assigned to HHMI.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
