Nanoparticle-Mediated Simultaneous Targeting of Mitochondrial Injury and Inflammation Attenuates Myocardial Ischemia-Reperfusion Injury
- PMID: 34056918
- PMCID: PMC8477875
- DOI: 10.1161/JAHA.120.019521
Nanoparticle-Mediated Simultaneous Targeting of Mitochondrial Injury and Inflammation Attenuates Myocardial Ischemia-Reperfusion Injury
Abstract
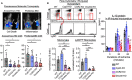
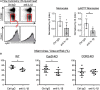
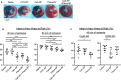
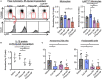
Background The opening of mitochondrial permeability transition pore and inflammation cooperatively progress myocardial ischemia-reperfusion (IR) injury, which hampers therapeutic effects of primary reperfusion therapy for acute myocardial infarction. We examined the therapeutic effects of nanoparticle-mediated medicine that simultaneously targets mitochondrial permeability transition pore and inflammation during IR injury. Methods and Results We used mice lacking cyclophilin D (CypD, a key molecule for mitochondrial permeability transition pore opening) and C-C chemokine receptor 2 and found that CypD contributes to the progression of myocardial IR injury at early time point (30-45 minutes) after reperfusion, whereas C-C chemokine receptor 2 contributes to IR injury at later time point (45-60 minutes) after reperfusion. Double deficiency of CypD and C-C chemokine receptor 2 enhanced cardioprotection compared with single deficiency regardless of the durations of ischemia. Deletion of C-C chemokine receptor 2, but not deletion of CypD, decreased the recruitment of Ly-6Chigh monocytes after myocardial IR injury. In CypD-knockout mice, administration of interleukin-1β blocking antibody reduced the recruitment of these monocytes. Combined administration of polymeric nanoparticles composed of poly-lactic/glycolic acid and encapsulating nanoparticles containing cyclosporine A or pitavastatin, which inhibit mitochondrial permeability transition pore opening and monocyte-mediated inflammation, respectively, augmented the cardioprotection as compared with single administration of nanoparticles containing cyclosporine A or pitavastatin after myocardial IR injury. Conclusions Nanoparticle-mediated simultaneous targeting of mitochondrial injury and inflammation could be a novel therapeutic strategy for the treatment of myocardial IR injury.
Keywords: cardioprotection; drug delivery system; ischemia‐reperfusion injury; nanotechnology.
Conflict of interest statement
None.
Figures


Similar articles
-
Nanoparticle-Mediated Targeting of Cyclosporine A Enhances Cardioprotection Against Ischemia-Reperfusion Injury Through Inhibition of Mitochondrial Permeability Transition Pore Opening.Sci Rep. 2016 Feb 10;6:20467. doi: 10.1038/srep20467. Sci Rep. 2016. PMID: 26861678 Free PMC article.
-
Nanoparticle incorporating Toll-like receptor 4 inhibitor attenuates myocardial ischaemia-reperfusion injury by inhibiting monocyte-mediated inflammation in mice.Cardiovasc Res. 2019 Jun 1;115(7):1244-1255. doi: 10.1093/cvr/cvz066. Cardiovasc Res. 2019. PMID: 30851101
-
Simultaneous targeting of mitochondria and monocytes enhances neuroprotection against ischemia-reperfusion injury.Sci Rep. 2020 Sep 2;10(1):14435. doi: 10.1038/s41598-020-71326-x. Sci Rep. 2020. PMID: 32879367 Free PMC article.
-
Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore.Circ J. 2013;77(5):1111-22. doi: 10.1253/circj.cj-13-0321. Epub 2013 Mar 29. Circ J. 2013. PMID: 23538482 Free PMC article. Review.
-
Targeting cell death.Clin Pharmacol Ther. 2007 Oct;82(4):370-3. doi: 10.1038/sj.clpt.6100352. Clin Pharmacol Ther. 2007. PMID: 17851576 Review.
Cited by
-
Molecular Signaling to Preserve Mitochondrial Integrity against Ischemic Stress in the Heart: Rescue or Remove Mitochondria in Danger.Cells. 2021 Nov 27;10(12):3330. doi: 10.3390/cells10123330. Cells. 2021. PMID: 34943839 Free PMC article. Review.
-
Dietary 7-ketocholesterol exacerbates myocardial ischemia-reperfusion injury in mice through monocyte/macrophage-mediated inflammation.Sci Rep. 2022 Sep 1;12(1):14902. doi: 10.1038/s41598-022-19065-z. Sci Rep. 2022. PMID: 36050346 Free PMC article.
-
Therapeutic Peptides to Treat Myocardial Ischemia-Reperfusion Injury.Front Cardiovasc Med. 2022 Feb 17;9:792885. doi: 10.3389/fcvm.2022.792885. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35252383 Free PMC article. Review.
-
Double Braking Effects of Nanomedicine on Mitochondrial Permeability Transition Pore for Treating Idiopathic Pulmonary Fibrosis.Adv Sci (Weinh). 2024 Dec;11(47):e2405406. doi: 10.1002/advs.202405406. Epub 2024 Oct 30. Adv Sci (Weinh). 2024. PMID: 39475000 Free PMC article.
-
Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders.Life (Basel). 2022 Apr 29;12(5):657. doi: 10.3390/life12050657. Life (Basel). 2022. PMID: 35629325 Free PMC article. Review.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. DOI: 10.1093/eurheartj/ehw128. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

