Plasma as a resuscitation fluid for volume-depleted shock: Potential benefits and risks
- PMID: 34057210
- PMCID: PMC8361764
- DOI: 10.1111/trf.16462
Plasma as a resuscitation fluid for volume-depleted shock: Potential benefits and risks
Keywords: FFP transfusion; blood management; plasma derivatives.
Conflict of interest statement
The author has disclosed no conflicts of interest.
Figures


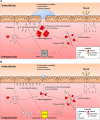

References
-
- Vincent J‐L, de Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–34. - PubMed
-
- Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277–93. - PubMed
-
- Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165:136–41. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

