Depletion of the gut microbiota differentially affects the impact of whey protein on high-fat diet-induced obesity and intestinal permeability
- PMID: 34057306
- PMCID: PMC8165735
- DOI: 10.14814/phy2.14867
Depletion of the gut microbiota differentially affects the impact of whey protein on high-fat diet-induced obesity and intestinal permeability
Abstract
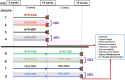
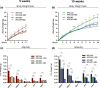
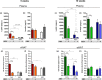
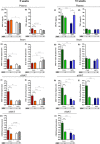
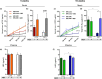
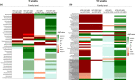
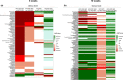
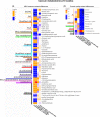
Whey protein isolate (WPI) is considered a dietary solution to obesity. However, the exact mechanism of WPI action is still poorly understood but is probably connected to its beneficial effect on energy balance, adiposity, and metabolism. More recently its ability to modulate the gut microbiota has received increasing attention. Here, we used a microbiota depletion, by antibiotic cocktail (ABX) administration, to investigate if the gut microbiota mediates the physiological and metabolic changes observed during high-fat diet (HFD)-WPI consumption. C57BL/6J mice received a HFD containing WPI (HFD-WPI) or the control non-whey milk protein casein (HFD-CAS) for 5 or 10 weeks. HFD-fed mice supplemented with WPI showed reduced body weight gain, adiposity, Ob gene expression level in the epidydimal adipose tissue (eWAT) and plasma leptin relative to HFD-CAS-fed mice, after 5- or 10-weeks intervention both with or without ABX treatment. Following 10-weeks intervention, ABX and WPI had an additive effect in lowering adiposity and leptin availability. HFD-WPI-fed mice showed a decrease in the expression of genes encoding pro-inflammatory markers (MCP-1, TNFα and CD68) within the ileum and eWAT, compared to HFD-CAS-fed mice, without showing alterations following microbiota depletion. Additionally, WPI supplementation decreased HFD-induced intestinal permeability disruption in the distal ileum; an effect that was reversed by chronic ABX treatment. In summary, WPI reverses the effects of HFD on metabolic and physiological functions through mainly microbiota-independent mechanisms. Moreover, we demonstrate a protective effect of WPI on HFD-induced inflammation and ileal permeability disruption, with the latter being reversed by gut microbiota depletion.
Keywords: adiposity; antibiotics; gut microbiota; gut permeability; high-fat diet; inflammation; metabolomics; whey protein.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Age- and duration-dependent effects of whey protein on high-fat diet-induced changes in body weight, lipid metabolism, and gut microbiota in mice.Physiol Rep. 2020 Aug;8(15):e14523. doi: 10.14814/phy2.14523. Physiol Rep. 2020. PMID: 32748559 Free PMC article.
-
Dietary α-lactalbumin alters energy balance, gut microbiota composition and intestinal nutrient transporter expression in high-fat diet-fed mice.Br J Nutr. 2019 May;121(10):1097-1107. doi: 10.1017/S0007114519000461. Epub 2019 Mar 5. Br J Nutr. 2019. PMID: 30834845
-
Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice.PLoS One. 2014 Feb 10;9(2):e88904. doi: 10.1371/journal.pone.0088904. eCollection 2014. PLoS One. 2014. PMID: 24520424 Free PMC article.
-
Targeting gut-liver axis by dietary lignans ameliorate obesity: evidences and mechanisms.Crit Rev Food Sci Nutr. 2025;65(2):243-264. doi: 10.1080/10408398.2023.2272269. Epub 2023 Oct 23. Crit Rev Food Sci Nutr. 2025. PMID: 37870876 Review.
-
Microbiota-Based Intervention Alleviates High-Fat Diet Consequences Through Host-Microbe Environment Remodeling.Nutrients. 2025 Apr 22;17(9):1402. doi: 10.3390/nu17091402. Nutrients. 2025. PMID: 40362711 Free PMC article. Review.
Cited by
-
Crocin-I Protects Against High-Fat Diet-Induced Obesity via Modulation of Gut Microbiota and Intestinal Inflammation in Mice.Front Pharmacol. 2022 Aug 11;13:894089. doi: 10.3389/fphar.2022.894089. eCollection 2022. Front Pharmacol. 2022. PMID: 36034852 Free PMC article.
-
Age matters: Microbiome depletion prior to repeat mild traumatic brain injury differentially alters microbial composition and function in adolescent and adult rats.PLoS One. 2022 Nov 30;17(11):e0278259. doi: 10.1371/journal.pone.0278259. eCollection 2022. PLoS One. 2022. PMID: 36449469 Free PMC article.
-
Intestinal permeability and peripheral immune cell composition are altered by pregnancy and adiposity at mid- and late-gestation in the mouse.PLoS One. 2023 Aug 7;18(8):e0284972. doi: 10.1371/journal.pone.0284972. eCollection 2023. PLoS One. 2023. PMID: 37549142 Free PMC article.
-
Age-related alterations in gut homeostasis are microbiota dependent.NPJ Biofilms Microbiomes. 2025 Mar 25;11(1):51. doi: 10.1038/s41522-025-00677-y. NPJ Biofilms Microbiomes. 2025. PMID: 40133348 Free PMC article.
-
The combination of berberine and evodiamine ameliorates high-fat diet-induced non-alcoholic fatty liver disease associated with modulation of gut microbiota in rats.Braz J Med Biol Res. 2022 May 16;55:e12096. doi: 10.1590/1414-431X2022e12096. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 35584453 Free PMC article.
References
-
- Araújo, J. R. , Tomas, J. , Brenner, C. , & Sansonetti, P. J. (2017). Impact of high‐fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie, 141, 97–106. - PubMed
-
- Aronesty, E. (2013). Comparison of sequencing utility programs. The Open Bioinformatics Journal, 7, 1–8.
-
- Benjamin, J. , Makharia, G. , Ahuja, V. , Anand Rajan, K. D. , Kalaivani, M. , Gupta, S. D. , & Joshi, Y. K. (2012). Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s Disease: A randomized controlled trial. Digestive Diseases and Sciences, 57(4), 1000–1012. - PubMed
-
- Bergia, R. E. 3rd , Hudson, J. L. , & Campbell, W. W. (2018). Effect of whey protein supplementation on body composition changes in women: A systematic review and meta‐analysis. Nutrition Reviews, 76(7), 539–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

