Plausible blockers of Spike RBD in SARS-CoV2-molecular design and underlying interaction dynamics from high-level structural descriptors
- PMID: 34057647
- PMCID: PMC8165686
- DOI: 10.1007/s00894-021-04779-0
Plausible blockers of Spike RBD in SARS-CoV2-molecular design and underlying interaction dynamics from high-level structural descriptors
Abstract
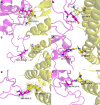
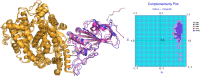
COVID-19 is characterized by an unprecedented abrupt increase in the viral transmission rate (SARS-CoV-2) relative to its pandemic evolutionary ancestor, SARS-CoV (2003). The complex molecular cascade of events related to the viral pathogenicity is triggered by the Spike protein upon interacting with the ACE2 receptor on human lung cells through its receptor binding domain (RBDSpike). One potential therapeutic strategy to combat COVID-19 could thus be limiting the infection by blocking this key interaction. In this current study, we adopt a protein design approach to predict and propose non-virulent structural mimics of the RBDSpike which can potentially serve as its competitive inhibitors in binding to ACE2. The RBDSpike is an independently foldable protein domain, resilient to conformational changes upon mutations and therefore an attractive target for strategic re-design. Interestingly, in spite of displaying an optimal shape fit between their interacting surfaces (attributed to a consequently high mutual affinity), the RBDSpike-ACE2 interaction appears to have a quasi-stable character due to a poor electrostatic match at their interface. Structural analyses of homologous protein complexes reveal that the ACE2 binding site of RBDSpike has an unusually high degree of solvent-exposed hydrophobic residues, attributed to key evolutionary changes, making it inherently "reaction-prone." The designed mimics aimed to block the viral entry by occupying the available binding sites on ACE2, are tested to have signatures of stable high-affinity binding with ACE2 (cross-validated by appropriate free energy estimates), overriding the native quasi-stable feature. The results show the apt of directly adapting natural examples in rational protein design, wherein, homology-based threading coupled with strategic "hydrophobic ↔ polar" mutations serve as a potential breakthrough.
Keywords: COVID-19; Competitive inhibitor; Complementarity; Homology-based threading in rational protein design; Protein design; SARS-CoV-2.
Conflict of interest statement
N/A
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

