Tacrolimus exposure windows responsible for Listeria monocytogenes infection susceptibility
- PMID: 34057792
- PMCID: PMC8455418
- DOI: 10.1111/tid.13655
Tacrolimus exposure windows responsible for Listeria monocytogenes infection susceptibility
Abstract
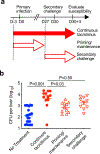
Tacrolimus is widely used to prevent graft rejection after allogeneic transplantation by suppressing T cells in a non-antigen-specific fashion. Global T-cell suppression makes transplant recipients more susceptible to infection, especially infection by opportunistic intracellular pathogens. Infection followed by secondary challenge with the opportunistic intracellular bacterial pathogen, Listeria monocytogenes, was used to probe when tacrolimus most significantly impacts antimicrobial host defense. Tacrolimus-treated mice showed no difference in innate susceptibility following primary infection, whereas susceptibility to secondary challenge was significantly increased. Modifying the timing of tacrolimus initiation with respect to primary infection compared with secondary challenge showed significantly reduced susceptibility in tacrolimus-treated mice where tacrolimus was discontinued prior to secondary challenge. Thus, tacrolimus overrides protection against secondary infection primed by primary infection (and presumably live attenuated vaccines), with the most critical window for tacrolimus-induced infection susceptibility being exposure immediately prior to secondary challenge. These results have important implications for strategies designed to boost antimicrobial T-cell-mediated immunity in transplant recipients.
Keywords: T cell; adaptive immunity; immune suppression; intracellular bacteria; opportunistic infection.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no conflicts interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

