Homocysteine induces podocyte apoptosis by regulating miR-1929-5p expression through c-Myc, DNMT1 and EZH2
- PMID: 34057794
- PMCID: PMC8564658
- DOI: 10.1002/1878-0261.13032
Homocysteine induces podocyte apoptosis by regulating miR-1929-5p expression through c-Myc, DNMT1 and EZH2
Abstract
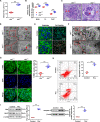
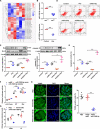
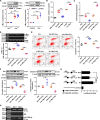
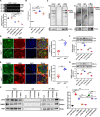
Chronic kidney disease (CKD) is a common and complex disease in kidneys which has been associated with an increased risk of renal cell carcinoma. Elevated homocysteine (Hcy) levels are known to influence the development and progression of CKD by regulating podocyte injury and apoptosis. To investigate the molecular mechanisms triggered in podocytes by Hcy, we used cbs+/- mice and observed that higher Hcy levels increased the apoptosis rate of podocytes with accompanying glomerular damage. Hcy-induced podocyte injury and apoptosis in cbs+/- mice was regulated by inhibition of microRNA (miR)-1929-5p expression. Overexpression of miR-1929-5p in podocytes inhibited apoptosis by upregulating Bcl-2. Furthermore, the expression of miR-1929-5p was regulated by epigenetic modifications of its promoter. Hcy upregulated DNA methyltransferase 1 (DNMT1) and enhancer of zeste homolog 2 (EZH2) levels, resulting in increased DNA methylation and H3K27me3 levels on the miR-1929-5p promoter. Additionally, we observed that c-Myc recruited DNMT1 and EZH2 to the miR-1929-5p promoter and suppressed the expression of miR-1929-5p. In summary, we demonstrated that Hcy promotes podocyte apoptosis through the regulation of the epigenetic modifiers DNMT1 and EZH2, which are recruited by c-Myc to the promoter of miR-1929-5p to silence miR-1929-5p expression.
Keywords: c-Myc; DNMT1; EZH2; homocysteine; kidney; miR-1929-5p.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Linehan WM & Ricketts CJ (2019) The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol 16, 539–552. - PubMed
-
- Ostrakhovitch EA & Tabibzadeh S (2015) Homocysteine in chronic kidney disease. Adv Clin Chem 72, 77–106. - PubMed
-
- Li S, Qiu B, Lu H, Lai Y, Liu J, Luo J, Zhu F, Hu Z, Zhou M, Tian J et al. (2019) Hyperhomocysteinemia accelerates acute kidney injury to chronic kidney disease progression by downregulating heme oxygenase‐1 expression. Antioxid Redox Signal 30, 1635–1650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

