Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort
- PMID: 34058148
- PMCID: PMC8570975
- DOI: 10.1016/S2213-2600(21)00079-5
Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort
Abstract
Background: We previously described the contributions of increased total airway mucin concentrations to the pathogenesis and diagnosis of the chronic bronchitic component of chronic obstructive pulmonary disease (COPD). Here, we investigated the relative contribution of each of the major airway gel-forming mucins, MUC5AC and MUC5B, to the initiation, progression, and early diagnosis of airways disease in COPD.
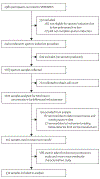
Methods: SPIROMICS was a multicentre, observational study in patients aged 40-80 years recruited from six clinical sites and additional subsites in the USA. In this analysis, MUC5AC and MUC5B were quantitated by stable isotope-labelled mass spectrometry in induced sputum samples from healthy never-smokers, ever-smokers at risk for COPD, and ever-smokers with COPD. Participants were extensively characterised using results from questionnaires, such as the COPD assessment test (CAT) and St George's Respiratory Questionnaire; quantitative CT, such as residual volume/total lung capacity ratio (RV/TLC) and parametric response mapping-functional small airway disease (PRM-fSAD); and pulmonary function tests, such as FEV1, forced vital capacity (FVC), and forced expiratory flow, midexpiratory phase (FEF25-75%). Absolute concentrations of both MUC5AC and MUC5B were related to cross-sectional (baseline, initial visit) and 3-year follow-up longitudinal data, including lung function, small airways obstruction, prospective acute exacerbations, and smoking status as primary outcomes. This study is registered with ClinicalTrials.gov (NCT01969344).
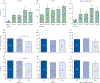
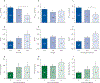
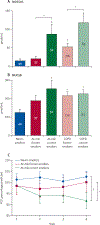
Findings: This analysis included 331 participants (mean age 63 years [SEM 9·40]), of whom 40 were healthy never-smokers, 90 were at-risk ever-smokers, and 201 were ever-smokers with COPD. Increased MUC5AC concentrations were more reliably associated with manifestations of COPD than were MUC5B concentrations, including decreased FEV1 and FEF25-75%, and increased prospective exacerbation frequency, RV/TLC, PRM-fSAD, and COPD assessment scores. MUC5AC concentrations were more reactive to cigarette smoke exposure than were MUC5B concentrations. Longitudinal data from 3-year follow-up visits generated a multivariate-adjusted odds ratio for two or more exacerbations of 1·24 (95% CI 1·04-1·47, p=0·015) for individuals with high baseline MUC5AC concentration. Increased MUC5AC, but not MUC5B, concentration at baseline was a significant predictor of FEV1, FEV1/FVC, FEF25-75%, and CAT score decline during the 3-year follow-up. Moreover, current smokers in the at-risk group showed raised MUC5AC concentrations at initial visits and decreased lung function over 3 years. By contrast, former smokers in the at-risk group showed normal MUC5AC concentrations at the initial visit and preserved lung function over 3 years.
Interpretation: These data indicate that increased MUC5AC concentration in the airways might contribute to COPD initiation, progression, exacerbation risk, and overall pathogenesis. Compared with MUC5B, greater relative changes in MUC5AC concentrations were observed as a function of COPD severity, and MUC5AC concentration seems to be an objective biomarker to detect disease in at-risk and pre-COPD individuals. These data suggest that MUC5AC-producing pathways could be potential targets for future therapeutic strategies. Thus, MUC5AC could be a novel biomarker for COPD prognosis and for testing the efficacy of therapeutic agents.
Funding: National Institutes of Health; National Heart, Lung, and Blood Institute.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests RGB reports grants from the National Institutes of Health (NIH), Foundation for the NIH (FNIH), and the COPD Foundation, during the study, and grants from NIH outside the submitted work. ERB has undertaken clinical trials through his employer, Wake Forest School of Medicine and University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson & Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme. ERB has also served as a paid consultant for AstraZeneca, MedImmune, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme, outside the submitted work. SAC reports personal fees from AstraZeneca, GlaxoSmithKline, Amgen, Glenmark, Sunovion, Genentech, and UpToDate, outside the submitted work. CBC reports grants from NIH/National Heart, Lung, and Blood Institute (NHLBI), FNIH, and COPD Foundation, during the conduct of the study. CBC reports personal fees from PulmonX, Nuvaira, and MGC Diagnostics, and is a Global Medical Expert for GlaxoSmithKline, outside the submitted work. MKH reports grants from NHLBI during the conduct of the study, personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Merck, Mylan, Teva, and Verona, and research support from Sanofi, Sunovion, and Novartis, outside the submitted work. NNH reports grants from NIH and COPD Foundation, grants and personal fees from AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim, and personal fees from Mylan, outside the submitted work. ATH reports grants from NHLBI and FNIH during the conduct of the study. EAH reports grants from NIH during the conduct of the study. EAH is a founder and shareholder of VIDA Diagnostics, outside the submitted work. FJM reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, and Raziel, during the conduct of the study. FJM reports personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Miller Communications, National Society for Continuing Education, PeerView Communications, Chiesi, Sunovion, Physicians Education Resource, Canadian Respiratory Network, Teva, CSL Behring, and Sanofi/Regeneron; non-financial support from ProterixBio, Gilead, Nitto, Zambon, and twoXAR; personal fees, non-financial support, and non-personnel travel support from Genentech; personal fees from MD Magazine, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape, Patara/Respivant, Bayer, American Thoracic Society, Rockpointe, CME Outfitters, Dartmouth University, DevPro, Gala, Integritas, IQVIA, Projects in Knowledge, Vindico, and Academy for Continuing Healthcare Learning; IPF Study Steering Committee for Afferent/Merck, Biogen, Veracyte, Prometic, and Bayer; IPF Advisor for Bridge Biotherapeutics; IPF teleconference with AbbVie; and grants from NIH, Rare Disease Healthcare Communications, and Promedior/Roche, outside the submitted work. RP reports grants from NHLBI and COPD Foundation, during the conduct of the study. RP reports grants from the Department of Veterans Affairs, and personal fees from Partner Therapeutics, outside the submitted work. PGW reports personal fees from Sanofi, Regeneron, Glenmark Pharmaceuticals, Theravance, GlaxoSmithKline, and NGM Pharma, outside the submitted work. WKO'N reports grants from NIH/NHLBI, during the conduct of the study. RCB reports grants from NIH, during the conduct of the study; and personal fees from Parion Sciences, outside the submitted work. MK reports grants from NIH, during the conduct of the study; contracts from Genentech, Gala Therapeutics, AstraZeneca, Ionis Pharmaceuticals, and personal fees from Boehringer Ingelheim and Amgen, outside the submitted work. In addition, MK has a patent (methods for diagnosing or predicting chronic bronchitis) pending. All other authors declare no competing interests.
Figures

References
-
- Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief 2011; 63: 1–8. - PubMed
-
- Rodriguez-Roisin R, Han MK, Vestbo J, Wedzicha JA, Woodruff PG, Martinez FJ. Chronic respiratory symptoms with normal spirometry. A reliable clinical entity? Am J Respir Crit Care Med 2017; 195: 17–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- P01 HL110873/HL/NHLBI NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- UH3 HL123645/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- R01 HL136961/HL/NHLBI NIH HHS/United States
- R01 HL103940/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- R01 HL110906/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- R01 HL135642/HL/NHLBI NIH HHS/United States
- P50 HL120100/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

