High frequency repetitive Transcranial Magnetic Stimulation promotes long lasting phrenic motoneuron excitability via GABAergic networks
- PMID: 34058433
- PMCID: PMC9447414
- DOI: 10.1016/j.resp.2021.103704
High frequency repetitive Transcranial Magnetic Stimulation promotes long lasting phrenic motoneuron excitability via GABAergic networks
Abstract
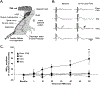
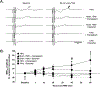
Repetitive transcranial magnetic stimulation (rTMS) is a promising, innovative, and non-invasive therapy used clinically. Efficacy of rTMS has been demonstrated to ameliorate psychiatric disorders and neuropathic pain through neuromodulation of affected neural circuits. However, little is known about the mechanisms and the specific neural circuits via which rTMS facilitates these functional effects. The aim of this study was to begin revealing the mechanisms by which rTMS may tap into existing neural circuits, by using a well characterized spinal motor circuit - the phrenic circuit. Here we hypothesized that rTMS can be used to enhance phrenic motoneuron excitability in anesthetized Sprague Dawley rats. Multiple acute rTMS protocols were used revealing 10 Hz rTMS protocol induced a robust, long-lasting increase in phrenic motoneuron excitability, functionally evaluated by diaphragm motor evoked potentials (59.1 ± 21.1 % of increase compared to baseline 60 min after 10 Hz protocol against 6.0 ± 5.8 % (p = 0.007) for Time Control, -5.8 ± 7.4 % (p < 0.001) for 3 Hz, and 5.2 ± 12.5 % (p = 0.008) for 30 Hz protocols). A deeper analyze allowed to discriminate "responder" and "non-responder" subgroups among 10 Hz rTMS treated animals. Intravenous injections of GABAA and GABAB receptor agonists prior to 10 Hz rTMS treatment, abolished the enhanced phrenic motoneuron excitability, suggesting GABAergic input plays a mechanistic role in rTMS-induced phrenic excitability. These data demonstrate that a single high frequency rTMS protocol at 10 Hz increases phrenic motoneuron excitability, mediated by a local GABAergic "disinhibition". By understanding how rTMS can be used to affect neural circuits non-invasively we can begin to harness the therapeutic potential of this neuromodulatory strategy to promote recovery after disease or injury to the central nervous system.
Keywords: GABAergic modulation; Motoneuron excitability; Preclinical model; rTMS.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
High-intensity, low-frequency repetitive transcranial magnetic stimulation enhances excitability of the human corticospinal pathway.J Neurophysiol. 2020 May 1;123(5):1969-1978. doi: 10.1152/jn.00607.2019. Epub 2020 Apr 15. J Neurophysiol. 2020. PMID: 32292098
-
Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation.J Neurophysiol. 2011 May;105(5):2150-6. doi: 10.1152/jn.00781.2010. Epub 2011 Feb 23. J Neurophysiol. 2011. PMID: 21346213 Clinical Trial.
-
Facilitatory conditioning of the supplementary motor area in humans enhances the corticophrenic responsiveness to transcranial magnetic stimulation.J Appl Physiol (1985). 2010 Jan;108(1):39-46. doi: 10.1152/japplphysiol.91454.2008. Epub 2009 Nov 5. J Appl Physiol (1985). 2010. PMID: 19892923
-
Releasing the Cortical Brake by Non-Invasive Electromagnetic Stimulation? rTMS Induces LTD of GABAergic Neurotransmission.Front Neural Circuits. 2016 Nov 28;10:96. doi: 10.3389/fncir.2016.00096. eCollection 2016. Front Neural Circuits. 2016. PMID: 27965542 Free PMC article. Review.
-
The effects of motor cortex rTMS on corticospinal descending activity.Clin Neurophysiol. 2010 Apr;121(4):464-73. doi: 10.1016/j.clinph.2009.11.007. Epub 2010 Jan 21. Clin Neurophysiol. 2010. PMID: 20096628 Review.
Cited by
-
Non-invasive Brain Stimulation for Central Neuropathic Pain.Front Mol Neurosci. 2022 May 19;15:879909. doi: 10.3389/fnmol.2022.879909. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35663263 Free PMC article. Review.
-
Repetitive Transcranial Magnetic Stimulation-Mediated Neuroprotection in the 5xFAD Mouse Model of Alzheimer's Disease Through GABRG2 and SNAP25 Modulation.Mol Neurobiol. 2025 Feb;62(2):1971-1997. doi: 10.1007/s12035-024-04354-7. Epub 2024 Jul 25. Mol Neurobiol. 2025. PMID: 39052185
-
Research progress on the application of transcranial magnetic stimulation in spinal cord injury rehabilitation: a narrative review.Front Neurol. 2023 Jul 18;14:1219590. doi: 10.3389/fneur.2023.1219590. eCollection 2023. Front Neurol. 2023. PMID: 37533475 Free PMC article. Review.
-
Novel role for non-invasive neuromodulation techniques in central respiratory dysfunction.Front Neurosci. 2023 Aug 23;17:1226660. doi: 10.3389/fnins.2023.1226660. eCollection 2023. Front Neurosci. 2023. PMID: 37680969 Free PMC article. Review.
-
Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats.Biology (Basel). 2022 Mar 19;11(3):473. doi: 10.3390/biology11030473. Biology (Basel). 2022. PMID: 35336846 Free PMC article.
References
-
- Alexandrov VG, Ivanova TG, Alexandrova NP, 2007. Prefrontal control of respiration. J. Physiol. Pharmacol 58 (Suppl 5), 17–23. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci 7, 48–55. - PubMed
-
- Benadhira R, Thomas F, Bouaziz N, Braha S, Andrianisaina PS, Isaac C, Moulier V, Januel D, 2017. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res. 258, 226–233. - PubMed
-
- Blandizzi C, Bernardini MC, Natale G, Martinotti E, Del Tacca M, 1992. Peripheral 2-hydroxy-saclofen-sensitive GABA-B receptors mediate both vagal-dependent and vagal-independent acid secretory responses in rats. J. Auton. Pharmacol 12, 149–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

