Evaluation of the Nature and Etiologies of Risk Factors for Diaphyseal Atypical Femoral Fractures
- PMID: 34058735
- PMCID: PMC8562049
- DOI: 10.1159/000517484
Evaluation of the Nature and Etiologies of Risk Factors for Diaphyseal Atypical Femoral Fractures
Abstract
Objectives: Differences in mechanisms of subtrochanteric and diaphyseal atypical femoral fractures (AFFs) are speculated in studies that analyzed differences in the patients' background. However, the etiologies of each type of AFF have not been studied in detail. This study aimed to investigate the nature and etiologies of the risk factors for diaphyseal AFFs.
Materials and methods: Eighty consecutive Japanese patients with 91 diaphyseal AFFs (AFF group) and 110 age-matched women with osteoporosis (non-AFF control group) were included. Their clinical data were compared; factors affecting AFFs were investigated, and the etiologies of the risk factors for diaphyseal AFFs were examined.
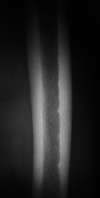
Results: Multivariate analysis revealed that femoral serrated changes, bisphosphonate or denosumab usage, and lateral and anterior femoral curvatures were risk factors for diaphyseal AFFs (p < 0.0011, p = 0.0137, and p < 0.0001, respectively). Multivariate analyses revealed that serrated changes and low serum 25(OH)D levels affected the lateral curvature (p = 0.0088 and 0.0205, respectively), while serrated changes affected the anterior curvature (p = 0.0006), each significantly affected the femoral curvature. High serum calcium (Ca) levels, lateral femoral curvature, and anterior femoral curvature were predictors of serrated changes (p = 0.0146, 0.0002, and 0.0098, respectively).
Conclusion: Risk factors for diaphyseal AFFs were bone resorption inhibitor usage, a strong femoral curvature, and serrated changes. Low serum 25(OH)D levels and serrated changes are risk factors for lateral curvature, while a high serum Ca level is a risk factor for serrated changes.
Keywords: Atypical femoral fracture; Femoral curvature; Serrated change.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Bamias A, Terpos E, Dimopoulos MA. Avascular osteonecrosis of the jaw as a side effect of bisphosphonate treatment. Onkologie. 2010;33((6)):288–9. - PubMed
-
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005 Mar;90((3)):1294–301. - PubMed
-
- Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011 May 5;364((18)):1728–37. - PubMed
-
- Feldstein AC, Black D, Perrin N, Rosales AG, Friess D, Boardman D, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res. 2012 May;27((5)):977–86. - PubMed
-
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med. 2012 Jun 25;172((12)):930–6. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical