The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism
- PMID: 34058787
- PMCID: PMC8239768
- DOI: 10.1113/EP089474
The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism
Abstract
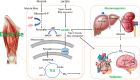
New findings: What is the topic of this review? Lactate is considered an important substrate for mitochondria in the muscles, heart and brain during exercise and is the main gluconeogenetic precursor in the liver and kidneys. In this light, we review the (patho)physiology of lactate metabolism in sepsis and coronavirus disease 2019 (COVID-19). What advances does it highlight? Elevated blood lactate is strongly associated with mortality in septic patients. Lactate seems unrelated to tissue hypoxia but is likely to reflect mitochondrial dysfunction and high adrenergic stimulation. Patients with severe COVID-19 exhibit near-normal blood lactate, indicating preserved mitochondrial function, despite a systemic hyperinflammatory state similar to sepsis.
Abstract: In critically ill patients, elevated plasma lactate is often interpreted as a sign of organ hypoperfusion and/or tissue hypoxia. This view on lactate is likely to have been influenced by the pioneering exercise physiologists around 1920. August Krogh identified an oxygen deficit at the onset of exercise that was later related to an oxygen 'debt' and lactate accumulation by A. V. Hill. Lactate is considered to be the main gluconeogenetic precursor in the liver and kidneys during submaximal exercise, but hepatic elimination is attenuated by splanchnic vasoconstriction during high-intensity exercise, causing an exponential increase in blood lactate. With the development of stable isotope tracers, lactate has become established as an important energy source for muscle, brain and heart tissue, where it is used for mitochondrial respiration. Plasma lactate > 4 mM is strongly associated with mortality in septic shock, with no direct link between lactate release and tissue hypoxia. Herein, we provide evidence for mitochondrial dysfunction and adrenergic stimulation as explanations for the sepsis-induced hyperlactataemia. Despite profound hypoxaemia and intense work of breathing, patients with severe coronavirus disease 2019 (COVID-19) rarely exhibit hyperlactataemia (> 2.5 mM), while presenting a systemic hyperinflammatory state much like sepsis. However, lactate dehydrogenase, which controls the formation of lactate, is markedly elevated in plasma and strongly associated with mortality in severe COVID-19. We briefly review the potential mechanisms of the lactate dehydrogenase elevation in COVID-19 and its relationship to lactate metabolism based on mechanisms established in contracting skeletal muscle and the acute respiratory distress syndrome.
Keywords: acute respiratory distress syndrome; cardiovascular system; critical care; exercise; lung injury.
© 2021 The Authors. Experimental Physiology © 2021 The Physiological Society.
Conflict of interest statement
None declared.
Figures

References
-
- Bassett, D. R. Jr. (2002). Scientific contributions of A. V. Hill: Exercise physiology pioneer. Journal of Applied Physiology, 93, 1567–1582. - PubMed
-
- Brooks, G. A. , Dubouchaud, H. , Brown, M. , Sicurello, J. P. , & Butz, C. E. (1999). Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proceedings of the National Academy of Sciences of the United States of America, 96, 1129–1134. 10.1073/pnas.96.3.1129 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

