Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors
- PMID: 34059088
- PMCID: PMC8165511
- DOI: 10.1186/s13045-021-01099-x
Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors
Abstract
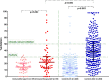
Vaccination for SARS-CoV-2 provides significant protection against the infection in the general population. However, only limited data exist for patients with cancer under systemic therapy. Based on this, our site has initiated a study evaluating safety and efficacy of SARS-CoV-2 vaccination in patients with solid and hematological malignancies under several systemic therapies. The initial results of the cohort of 59 patients receiving Immune Checkpoint Inhibitors are presented here. Despite no new safety issues have been noticed, the levels of SARS-CoV-2 neutralizing antibodies are significantly lower in comparison to matched healthy volunteers up to day 22 post the first dose. These results should be taken into consideration for the patients under treatment.
Keywords: AZD1222; BNT162b2; Cancer; Immune checkpoint inhibitors; SARS-CoV-2; Vaccination.
Conflict of interest statement
We declare no competing interests.
Figures
References
-
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

