Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke
- PMID: 34059091
- PMCID: PMC8166383
- DOI: 10.1186/s12974-021-02137-8
Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke
Abstract
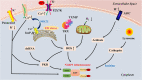
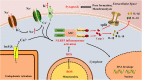
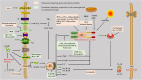
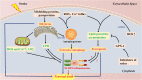
The nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome is a member of the NLR family of inherent immune cell sensors. The NLRP3 inflammasome can detect tissue damage and pathogen invasion through innate immune cell sensor components commonly known as pattern recognition receptors (PRRs). PRRs promote activation of nuclear factor kappa B (NF-κB) pathways and the mitogen-activated protein kinase (MAPK) pathway, thus increasing the transcription of genes encoding proteins related to the NLRP3 inflammasome. The NLRP3 inflammasome is a complex with multiple components, including an NAIP, CIITA, HET-E, and TP1 (NACHT) domain; apoptosis-associated speck-like protein containing a CARD (ASC); and a leucine-rich repeat (LRR) domain. After ischemic stroke, the NLRP3 inflammasome can produce numerous proinflammatory cytokines, mediating nerve cell dysfunction and brain edema and ultimately leading to nerve cell death once activated. Ischemic stroke is a disease with high rates of mortality and disability worldwide and is being observed in increasingly younger populations. To date, there are no clearly effective therapeutic strategies for the clinical treatment of ischemic stroke. Understanding the NLRP3 inflammasome may provide novel ideas and approaches because targeting of upstream and downstream molecules in the NLRP3 pathway shows promise for ischemic stroke therapy. In this manuscript, we summarize the existing evidence regarding the composition and activation of the NLRP3 inflammasome, the molecules involved in inflammatory pathways, and corresponding drugs or molecules that exert effects after cerebral ischemia. This evidence may provide possible targets or new strategies for ischemic stroke therapy.
Keywords: Inflammation; Ischemic stroke; NLRP3 inflammasome; Reactive oxygen species; Signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Evidence that NF-κB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke.Mol Neurobiol. 2018 Feb;55(2):1082-1096. doi: 10.1007/s12035-017-0394-9. Epub 2017 Jan 14. Mol Neurobiol. 2018. PMID: 28092085
-
Neuroinflammation and COVID-19 Ischemic Stroke Recovery-Evolving Evidence for the Mediating Roles of the ACE2/Angiotensin-(1-7)/Mas Receptor Axis and NLRP3 Inflammasome.Int J Mol Sci. 2022 Mar 13;23(6):3085. doi: 10.3390/ijms23063085. Int J Mol Sci. 2022. PMID: 35328506 Free PMC article. Review.
-
Vinpocetine Attenuates Ischemic Stroke Through Inhibiting NLRP3 Inflammasome Expression in Mice.J Cardiovasc Pharmacol. 2020 Dec 22;77(2):208-216. doi: 10.1097/FJC.0000000000000945. J Cardiovasc Pharmacol. 2020. PMID: 33351536 Free PMC article.
Cited by
-
Molecular Mechanisms of Inflammasome in Ischemic Stroke Pathogenesis.Pharmaceuticals (Basel). 2022 Sep 21;15(10):1168. doi: 10.3390/ph15101168. Pharmaceuticals (Basel). 2022. PMID: 36297283 Free PMC article. Review.
-
Prevalence and Related Factors of Hypokalemia in Patients with Acute Ischemic Stroke.Int J Gen Med. 2024 Nov 30;17:5697-5705. doi: 10.2147/IJGM.S492025. eCollection 2024. Int J Gen Med. 2024. PMID: 39635664 Free PMC article.
-
A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway.Brain Sci. 2024 Jul 31;14(8):781. doi: 10.3390/brainsci14080781. Brain Sci. 2024. PMID: 39199474 Free PMC article.
-
Endoplasmic Reticulum Stress and the Unfolded Protein Response in Cerebral Ischemia/Reperfusion Injury.Front Cell Neurosci. 2022 May 4;16:864426. doi: 10.3389/fncel.2022.864426. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35602556 Free PMC article. Review.
-
Identification of Hub Genes Associated with Immune Infiltration in Cardioembolic Stroke by Whole Blood Transcriptome Analysis.Dis Markers. 2022 Jan 15;2022:8086991. doi: 10.1155/2022/8086991. eCollection 2022. Dis Markers. 2022. PMID: 35075378 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

