NIPU: a randomised, open-label, phase II study evaluating nivolumab and ipilimumab combined with UV1 vaccination as second line treatment in patients with malignant mesothelioma
- PMID: 34059094
- PMCID: PMC8165504
- DOI: 10.1186/s12967-021-02905-3
NIPU: a randomised, open-label, phase II study evaluating nivolumab and ipilimumab combined with UV1 vaccination as second line treatment in patients with malignant mesothelioma
Abstract
Background: Malignant pleural mesothelioma (MPM) is a rare and aggressive tumour. For patients with inoperable disease, few treatment options are available after first line chemotherapy. The combination of ipilimumab and nivolumab has recently shown increased survival compared to standard chemotherapy, but most patients do not respond and improvements are called for. Telomerase is expressed in mesothelioma cells, but only sparsely in normal tissues and is therefore an attractive target for therapeutic vaccination. Vaccination against telomerase is tolerable and has shown to induce immune responses associated with increased survival in other cancer types. There is a well-founded scientific rationale for the combination of a telomerase vaccine and checkpoint inhibition to improve treatment response in MPM patients.
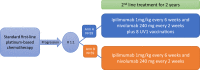
Methods: NIPU is a randomized, multi-centre, open-label, phase II study comparing the efficacy and safety of nivolumab and ipilimumab with or without telomerase vaccine in patients with inoperable malignant pleural mesothelioma after first-line platinum-based chemotherapy. Participants (n = 118) are randomized 1:1 into two treatment arms. All participants receive treatment with nivolumab (240 mg every 2 weeks) and ipilimumab (1 mg/kg every 6 weeks) until disease progression, unacceptable toxicity or for a maximum of 2 years. Patients randomised to the experimental arm receive 8 intradermal injections of UV1 vaccine during the first three months of treatment. Tumour tissue, blood, urine, faeces and imaging will be collected for biomarker analyses and exploration of mechanisms for response and resistance to therapy.
Discussion: Checkpoint inhibition is used for treatment of mesothelioma, but many patients still do not respond. Increasing therapy response to immunotherapy is an important goal. Possible approaches include combination with chemotherapy, radiotherapy, targeted therapy and other immunotherapeutic agents. Predictive biomarkers are necessary to ensure optimal treatment for each patient and to prevent unnecessary side effects. This trial seeks to improve treatment response by combining checkpoint inhibition with a telomerase vaccine and also to explore mechanisms for treatment response and resistance. Knowledge gained in the NIPU study may be transferred to the first line setting and to other cancers with limited benefit from immunotherapy.
Trial registration: ClinicalTrials.gov: NCT04300244, registered March 8th, 2020, https://clinicaltrials.gov/ct2/show/NCT04300244?term=NIPU&draw=2&rank=1 .
Keywords: Biomarker; Immune response; Immunotherapy; Ipilimumab; Malignant pleural mesothelioma; Nivolumab; Telomerase vaccine; hTERT.
Conflict of interest statement
EBE is employed by Ultimovacs ASA. AKN has participated as a consultant for Bristol Meyers Squibb. The remaining authors declare that they have no competing interests.
Figures
References
-
- Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma ( KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical