Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients
- PMID: 34059198
- PMCID: PMC8169123
- DOI: 10.7554/eLife.58775
Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients
Abstract
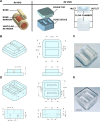
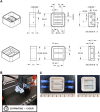
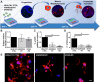
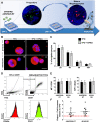
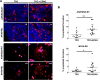
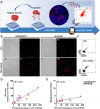
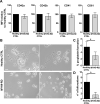
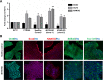
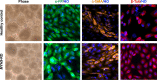
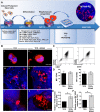
Thrombocytopenic disorders have been treated with the Thrombopoietin-receptor agonist Eltrombopag. Patients with the same apparent form of thrombocytopenia may respond differently to the treatment. We describe a miniaturized bone marrow tissue model that provides a screening bioreactor for personalized, pre-treatment response prediction to Eltrombopag for individual patients. Using silk fibroin, a 3D bone marrow niche was developed that reproduces platelet biogenesis. Hematopoietic progenitors were isolated from a small amount of peripheral blood of patients with mutations in ANKRD26 and MYH9 genes, who had previously received Eltrombopag. The ex vivo response was strongly correlated with the in vivo platelet response. Induced Pluripotent Stem Cells (iPSCs) from one patient with mutated MYH9 differentiated into functional megakaryocytes that responded to Eltrombopag. Combining patient-derived cells and iPSCs with the 3D bone marrow model technology allows having a reproducible system for studying drug mechanisms and for individualized, pre-treatment selection of effective therapy in Inherited Thrombocytopenias.
Keywords: Induced Pluripotent Stem Cell; Inherited Thrombocytopenia; bone marrow; human; medicine; megakaryocyte; silk; thrombopoietin.
Plain language summary
Platelets are tiny cell fragments essential for blood to clot. They are created and released into the bloodstream by megakaryocytes, giant cells that live in the bone marrow. In certain genetic diseases, such as Inherited Thrombocytopenia, the bone marrow fails to produce enough platelets: this leaves patients extremely susceptible to bruising, bleeding, and poor clotting after an injury or surgery. Certain patients with Inherited Thrombocytopenia respond well to treatments designed to boost platelet production, but others do not. Why these differences exist could be investigated by designing new test systems that recreate the form and function of bone marrow in the laboratory. However, it is challenging to build the complex and poorly understood bone marrow environment outside of the body. Here, Di Buduo et al. have developed an artificial three-dimensional miniature organ bioreactor system that recreates the key features of bone marrow. In this system, megakaryocytes were grown from patient blood samples, and hooked up to a tissue scaffold made of silk. The cells were able to grow as if they were in their normal environment, and they could shed platelets into an artificial bloodstream. After treating megakaryocytes with drugs to stimulate platelet production, Di Buduo et al. found that the number of platelets recovered from the bioreactor could accurately predict which patients would respond to these drugs in the clinic. This new test system enables researchers to predict how a patient will respond to treatment, and to tailor therapy options to each individual. This technology could also be used to test new drugs for Inherited Thrombocytopenias and other blood-related diseases; if scaled-up, it could also, one day, generate large quantities of lab-grown blood cells for transfusion.
© 2021, Di Buduo et al.
Conflict of interest statement
CD, PL, CZ, LL, PS, AN, SB, AL, IB, HR, DK, CB, AP, AB No competing interests declared, JB James B Bussel is consultant and participant in advisory boards for Amgen, Novartis, Dova, Rigel, UCB, Argenx, Momenta, Regeneron
Figures






Similar articles
-
Revealing eltrombopag's promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation.Haematologica. 2016 Dec;101(12):1479-1488. doi: 10.3324/haematol.2016.146746. Epub 2016 Aug 11. Haematologica. 2016. PMID: 27515246 Free PMC article.
-
Eltrombopag in chronic hepatitis C.World J Gastroenterol. 2014 Sep 21;20(35):12517-21. doi: 10.3748/wjg.v20.i35.12517. World J Gastroenterol. 2014. PMID: 25253952 Free PMC article. Review.
-
Humanized VB22B minibody for human Mpl stimulates human megakaryopoiesis but does not enhance platelet aggregation.Exp Hematol. 2011 Aug;39(8):829-36. doi: 10.1016/j.exphem.2011.05.001. Epub 2011 May 7. Exp Hematol. 2011. PMID: 21605620
-
Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations.Blood. 2010 Dec 23;116(26):5832-7. doi: 10.1182/blood-2010-08-304725. Epub 2010 Sep 15. Blood. 2010. PMID: 20844233 Clinical Trial.
-
Thrombopoietin receptor agonists in hereditary thrombocytopenias.J Thromb Haemost. 2018 Sep;16(9):1700-1710. doi: 10.1111/jth.14217. Epub 2018 Jul 27. J Thromb Haemost. 2018. PMID: 29956472 Review.
Cited by
-
Immune attack on megakaryocytes in immune thrombocytopenia.Res Pract Thromb Haemost. 2024 Mar 14;8(1):102345. doi: 10.1016/j.rpth.2024.102345. eCollection 2024 Jan. Res Pract Thromb Haemost. 2024. PMID: 38525349 Free PMC article.
-
Inside-to-outside and back to the future of megakaryopoiesis.Res Pract Thromb Haemost. 2023 May 30;7(4):100197. doi: 10.1016/j.rpth.2023.100197. eCollection 2023 May. Res Pract Thromb Haemost. 2023. PMID: 37416054 Free PMC article.
-
A millifluidic bioreactor allows the long term culture of primary lymphocytes or CD34+ hematopoietic cells while allowing the detection of tumorigenic expansion.Front Bioeng Biotechnol. 2024 Oct 2;12:1388312. doi: 10.3389/fbioe.2024.1388312. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416278 Free PMC article.
-
Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells.Int J Mol Sci. 2022 Apr 18;23(8):4462. doi: 10.3390/ijms23084462. Int J Mol Sci. 2022. PMID: 35457280 Free PMC article. Review.
-
Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy.Biomedicines. 2023 Aug 10;11(8):2244. doi: 10.3390/biomedicines11082244. Biomedicines. 2023. PMID: 37626740 Free PMC article. Review.
References
-
- Abbonante V, Di Buduo CA, Gruppi C, Malara A, Gianelli U, Celesti G, Anselmo A, Laghi L, Vercellino M, Visai L, Iurlo A, Moratti R, Barosi G, Rosti V, Balduini A. Thrombopoietin/TGF-β1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells. 2016;34:1123–1133. doi: 10.1002/stem.2285. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

