Single molecule FRET methodology for investigating glutamate receptors
- PMID: 34059282
- PMCID: PMC8215891
- DOI: 10.1016/bs.mie.2021.02.005
Single molecule FRET methodology for investigating glutamate receptors
Abstract
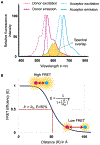
Single molecule Förster Resonance Energy Transfer (smFRET) allows us to measure variation in distances between donor and acceptor fluorophores attached to a protein, providing the conformational landscape of the protein with respect to this specific distance. smFRET can be performed on freely diffusing molecules or on tethered molecules. Here, we describe the tethered method used to study ionotropic glutamate receptors, which allows us to track the changes in FRET as a function of time, thus providing information on the conformations sampled and kinetics of conformational changes in the millisecond to second time scale. Strategies for attaching fluorophores to the proteins, methods for acquiring and analyzing the smFRET trajectories, and limitations are discussed.
Keywords: Conformational landscape; Efficiency histograms; Fluorophores; Ionotropic glutamate receptors; smFRET.
© 2021 Elsevier Inc. All rights reserved.
Figures


References
-
- Aggarwal V, & Ha T, (2014). Single-molecule pull-down (SiMPull) for new-age biochemistry: methodology and biochemical applications of single-molecule pull-down (SiMPull) for probing biomolecular interactions in crude cell extracts. Bioessays, 36(11), 1109–1119. doi:10.1002/bies.201400090 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

