A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity
- PMID: 34059617
- PMCID: PMC8165147
- DOI: 10.1038/s41392-021-00634-z
A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity
Abstract
Although inoculation of COVID-19 vaccines has rolled out globally, there is still a critical need for safe and effective vaccines to ensure fair and equitable supply for all countries. Here, we report on the development of a highly efficacious mRNA vaccine, SW0123 that is composed of sequence-modified mRNA encoding the full-length SARS-CoV-2 Spike protein packaged in core-shell structured lipopolyplex (LPP) nanoparticles. SW0123 is easy to produce using a large-scale microfluidics-based apparatus. The unique core-shell structured nanoparticle facilitates vaccine uptake and demonstrates a high colloidal stability, and a desirable biodistribution pattern with low liver targeting effect upon intramuscular administration. Extensive evaluations in mice and nonhuman primates revealed strong immunogenicity of SW0123, represented by induction of Th1-polarized T cell responses and high levels of antibodies that were capable of neutralizing not only the wild-type SARS-CoV-2, but also a panel of variants including D614G and N501Y variants. In addition, SW0123 conferred effective protection in both mice and non-human primates upon SARS-CoV-2 challenge. Taken together, SW0123 is a promising vaccine candidate that holds prospects for further evaluation in humans.
Conflict of interest statement
Stemirna Therapeutics filed patents on patent application entitled “A prophylactic or therapeutical 2019-nCoV mRNA vaccine”. All other authors declare no conflict of interest.
Figures
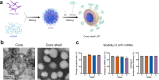
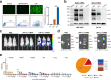
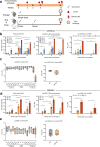
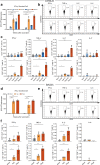
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

