Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models
- PMID: 34059622
- PMCID: PMC8165351
- DOI: 10.1038/s41420-021-00510-3
Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models
Abstract
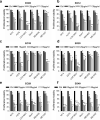
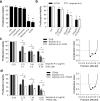
Essential oils (EOs) have been recently emerging for their promising biological activities in preventing tumorigenesis or progression of different tumor histotypes, including melanoma. In this study, we investigated the antitumor activity of a panel of EOs in different tumor models. The ability of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol, to sensitize the target therapy currently used for melanoma treatment was also assessed. Our results demonstrated that EOs differently affect the viability of human cancer cells and led us to select six EOs effective in melanoma and lung cancer cells, without toxic effects in human fibroblasts. When combined with dabrafenib and/or trametinib, Melaleuca alternifolia synergistically reduced the viability of melanoma cells by activating apoptosis. Through machine learning classification modeling, α-terpineol, tepinolene, and terpinen-4-ol, three components of Melaleuca alternifolia, were identified as the most likely relevant components responsible for the EO's antitumor effect. Among them, terpinen-4-ol was recognized as the Melaleuca alternifolia component responsible for its antitumor and proapoptotic activity. Overall, our study holds promise for further analysis of EOs as new anticancer agents and supports the rationale for their use to improve target therapy response in melanoma.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Flaherty LE, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma-an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J. Clin. Oncol. 2014;32:3771–3778. doi: 10.1200/JCO.2013.53.1590. - DOI - PMC - PubMed
Grants and funding
- Ateneo Grant 2019- P.I. RR (prot. RM11916B8876093E)/Sapienza Università di Roma (Sapienza University of Rome)
- Ateneo Grant 2018-P.I. RR (prot. RM118164361B425B)/Sapienza Università di Roma (Sapienza University of Rome)
- IG 2020 - ID. 24315/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
LinkOut - more resources
Full Text Sources

